A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward
- PMID: 31348890
- PMCID: PMC7001890
- DOI: 10.1016/j.cell.2019.06.034
A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward
Abstract
Nociceptin and its receptor are widely distributed throughout the brain in regions associated with reward behavior, yet how and when they act is unknown. Here, we dissected the role of a nociceptin peptide circuit in reward seeking. We generated a prepronociceptin (Pnoc)-Cre mouse line that revealed a unique subpopulation of paranigral ventral tegmental area (pnVTA) neurons enriched in prepronociceptin. Fiber photometry recordings during progressive ratio operant behavior revealed pnVTAPnoc neurons become most active when mice stop seeking natural rewards. Selective pnVTAPnoc neuron ablation, inhibition, and conditional VTA nociceptin receptor (NOPR) deletion increased operant responding, revealing that the pnVTAPnoc nucleus and VTA NOPR signaling are necessary for regulating reward motivation. Additionally, optogenetic and chemogenetic activation of this pnVTAPnoc nucleus caused avoidance and decreased motivation for rewards. These findings provide insight into neuromodulatory circuits that regulate motivated behaviors through identification of a previously unknown neuropeptide-containing pnVTA nucleus that limits motivation for rewards.
Keywords: NOPR; motivation; neuropeptide; nociceptin; opioid; optogenetics; orphanin FQ; photometry; reward; ventral tegmental area.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
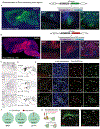
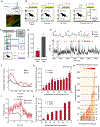

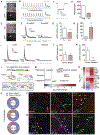

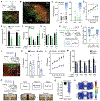

Comment in
-
Neuropeptide regulation of motivation.Nat Rev Neurosci. 2019 Oct;20(10):574-575. doi: 10.1038/s41583-019-0214-5. Nat Rev Neurosci. 2019. PMID: 31391547 No abstract available.
-
Ring of Power: A Band of Peptidergic Midbrain Neurons that Binds Motivation.Neuron. 2019 Aug 7;103(3):364-366. doi: 10.1016/j.neuron.2019.07.022. Neuron. 2019. PMID: 31394061
References
-
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL,, Rogers JA, Bruchas MR (2015). Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron, 87, 1063–1077. - PMC - PubMed
-
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ (1996). Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol. 368(2), 229–251. - PubMed
-
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB (2001). Neurotensin and dopamine interactions. Pharmacol Rev, 53, 453–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DA033396/DA/NIDA NIH HHS/United States
- F32 DA041184/DA/NIDA NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- T32 DA007261/DA/NIDA NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 DA038168/DA/NIDA NIH HHS/United States
- R21 DA034929/DA/NIDA NIH HHS/United States
- T32 EB014855/EB/NIBIB NIH HHS/United States
- T32 GM008151/GM/NIGMS NIH HHS/United States
- R00 DA038725/DA/NIDA NIH HHS/United States
- P30 DA048736/DA/NIDA NIH HHS/United States
- U01 MH109133/MH/NIMH NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R37 DA032750/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

