Sodium homeostasis in the tumour microenvironment
- PMID: 31348974
- PMCID: PMC7115894
- DOI: 10.1016/j.bbcan.2019.07.001
Sodium homeostasis in the tumour microenvironment
Abstract
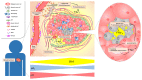
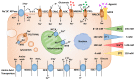
The concentration of sodium ions (Na+) is raised in solid tumours and can be measured at the cellular, tissue and patient levels. At the cellular level, the Na+ gradient across the membrane powers the transport of H+ ions and essential nutrients for normal activity. The maintenance of the Na+ gradient requires a large proportion of the cell's ATP. Na+ is a major contributor to the osmolarity of the tumour microenvironment, which affects cell volume and metabolism as well as immune function. Here, we review evidence indicating that Na+ handling is altered in tumours, explore our current understanding of the mechanisms that may underlie these alterations and consider the potential consequences for cancer progression. Dysregulated Na+ balance in tumours may open opportunities for new imaging biomarkers and re-purposing of drugs for treatment.
Keywords: Channels; MRI; Microenvironment; Sodium; Transporters; Tumours.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
JDK reports grants from GSK, EU2020, and Cambridge Biomedical Research Centre, outside the submitted work. FAG reports grants from GSK and non-financial support from GE Healthcare. FDG reports grants from GE healthcare, personal fees from Google DeepMind, and non-financial support from Bracco, outside the submitted work. WJB reports grants from Cancer Research UK and Breast Cancer Now.
Figures


References
-
- Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671–7. - PubMed
-
- Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14(1):39–48. - PubMed
-
- Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium–cancer signalling nexus. Nat Rev Cancer. 2017;17(6):367–80. - PubMed
-
- Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology. 2003;227(2):529. - PubMed
-
- Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16(3):107–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

