Towards a nanoparticle-based prophylactic for maternal autoantibody-related autism
- PMID: 31349087
- PMCID: PMC7197945
- DOI: 10.1016/j.nano.2019.102067
Towards a nanoparticle-based prophylactic for maternal autoantibody-related autism
Abstract
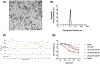
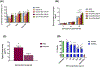
Recently, the causative agents of Maternal Autoantibody-Related (MAR) autism, pathological autoantibodies and their epitopic targets (e.g. lactate dehydrogenase B [LDH B] peptide), have been identified. Herein, we report on the development of Systems for Nanoparticle-based Autoantibody Reception and Entrapment (SNAREs), which we hypothesized could scavenge disease-propagating MAR autoantibodies from the maternal blood. To demonstrate this functionality, we synthesized 15 nm dextran iron oxide nanoparticles surface-modified with citric acid, methoxy PEG(10 kDa) amine, and LDH B peptide (33.8 μg peptide/cm2). In vitro, we demonstrated significantly lower macrophage uptake for SNAREs compared to control NPs. The hallmark result of this study was the efficacy of the SNAREs to remove 90% of LDH B autoantibody from patient-derived serum. Further, in vitro cytotoxicity testing and a maximal tolerated dose study in mice demonstrated the safety of the SNARE formulation. This work establishes the feasibility of SNAREs as the first-ever prophylactic against MAR autism.
Keywords: Iron oxide(2); Maternal autoantibody-related autism(1); Peptide-functionalized(4); nanoparticles(3).
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: Dr. Van de Water has a patent application involving the MAR ASD peptides described herein; all other authors have no conflicts of interest to declare.
Figures
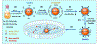

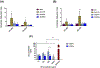


References
-
- DSM-5 autism spectrum disorder fact sheet. Association AP. 2013.
-
- Christensen DL, Baio J, Van Naarden Braun K., Bilder D, Charles J, Constantino JN, et al. Prevalence and characteristics of autism Spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012 MMWR Surveill Summ, 65 (3) (2016), pp. 1–23 - PMC - PubMed
-
- Leigh JP, Du J. Brief report: forecasting the economic burden of autism in 2015 and 2025 in the United States J Autism Dev Disord, 45 (12) (2015), pp. 4135–4139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

