Novel Markers of the Metabolic Impact of Exogenous Retinoic Acid with A Focus on Acylcarnitines and Amino Acids
- PMID: 31349613
- PMCID: PMC6696161
- DOI: 10.3390/ijms20153640
Novel Markers of the Metabolic Impact of Exogenous Retinoic Acid with A Focus on Acylcarnitines and Amino Acids
Abstract
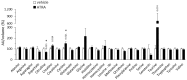
Treatment with all-trans retinoic acid (ATRA), the carboxylic form of vitamin A, lowers body weight in rodents by promoting oxidative metabolism in multiple tissues including white and brown adipose tissues. We aimed to identify novel markers of the metabolic impact of ATRA through targeted blood metabolomics analyses, with a focus on acylcarnitines and amino acids. Blood was obtained from mice treated with a high ATRA dose (50 mg/kg body weight/day, subcutaneous injection) or placebo (controls) during the 4 days preceding collection. LC-MS/MS analyses with a focus on acylcarnitines and amino acids were conducted on plasma and PBMC. Main results showed that, relative to controls, ATRA-treated mice had in plasma: increased levels of carnitine, acetylcarnitine, and longer acylcarnitine species; decreased levels of citrulline, and increased global arginine bioavailability ratio for nitric oxide synthesis; increased levels of creatine, taurine and docosahexaenoic acid; and a decreased n-6/n-3 polyunsaturated fatty acids ratio. While some of these features likely reflect the stimulation of lipid mobilization and oxidation promoted by ATRA treatment systemically, other may also play a causal role underlying ATRA actions. The results connect ATRA to specific nutrition-modulated biochemical pathways, and suggest novel mechanisms of action of vitamin A-derived retinoic acid on metabolic health.
Keywords: acylcarnitines; amino acids; retinoic acid; targeted metabolomics; vitamin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Alvarez R., de Andres J., Yubero P., Vinas O., Mampel T., Iglesias R., Giralt M., Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 1995;270:5666–5673. doi: 10.1074/jbc.270.10.5666. - DOI - PubMed
-
- Bonet M.L., Oliver J., Pico C., Felipe F., Ribot J., Cinti S., Palou A. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J. Endocrinol. 2000;166:511–517. doi: 10.1677/joe.0.1660511. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

