Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms
- PMID: 31349628
- PMCID: PMC6783858
- DOI: 10.3390/antibiotics8030103
Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms
Abstract
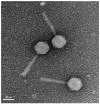
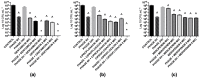
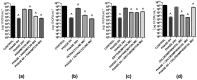
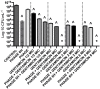
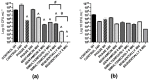
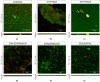
Pseudomonas aeruginosa and Staphylococcus aureus are opportunistic pathogens and are commonly found in polymicrobial biofilm-associated diseases, namely chronic wounds. Their co-existence in a biofilm contributes to an increased tolerance of the biofilm to antibiotics. Combined treatments of bacteriophages and antibiotics have shown a promising antibiofilm activity, due to the profound differences in their mechanisms of action. In this study, 48 h old mono and dual-species biofilms were treated with a newly isolated P. aeruginosa infecting phage (EPA1) and seven different antibiotics (gentamicin, kanamycin, tetracycline, chloramphenicol, erythromycin, ciprofloxacin, and meropenem), alone and in simultaneous or sequential combinations. The therapeutic efficacy of the tested antimicrobials was determined. Phage or antibiotics alone had a modest effect in reducing biofilm bacteria. However, when applied simultaneously, a profound improvement in the killing effect was observed. Moreover, an impressive biofilm reduction (below the detection limit) was observed when gentamicin or ciprofloxacin were added sequentially after 6 h of phage treatment. The effect observed does not depend on the type of antibiotic but is influenced by its concentration. Moreover, in dual-species biofilms it was necessary to increase gentamicin concentration to obtain a similar killing effect as occurs in mono-species. Overall, combining phages with antibiotics can be synergistic in reducing the bacterial density in biofilms. However, the concentration of antibiotic and the time of antibiotic application are essential factors that need to be considered in the combined treatments.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; antibiotic; bacteriophage; biofilms; dual-species; sequential; simultaneous; synergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
- UID/BIO/04469/2013/Fundação para a Ciência e a Tecnologia
- PTDC/BBB-BSS/6471/2014/Fundação para a Ciência e a Tecnologia
- POCI-01-0145-FEDER-006684/Fundação para a Ciência e a Tecnologia
- NORTE-01-0145-FEDER-000004/European Regional Development Fund
- PD/BD/135254/2017/Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources
Other Literature Sources

