Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception
- PMID: 31349649
- PMCID: PMC6789544
- DOI: 10.3390/vaccines7030073
Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception
Abstract
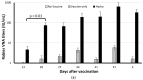
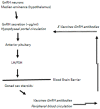
Rabies is preventable through vaccination, but the need to mount annual canine vaccination campaigns presents major challenges in rabies control and prevention. The development of a rabies vaccine that ensures lifelong immunity and animal population management in one dose could be extremely advantageous. A nonsurgical alternative to spay/neuter is a high priority for animal welfare, but irreversible infertility in one dose has not been achieved. Towards this goal, we developed a rabies virus-vectored immunocontraceptive vaccine ERA-2GnRH, which protected against rabies virus challenge and induced >80% infertility in mice after three doses in a live, liquid-vaccine formulation (Wu et al., 2014). To improve safety and use, we formulated an inactivated vaccine in a thermo-responsive chitosan hydrogel for one-dose delivery and studied the immune responses in mice. The hydrogel did not cause any injection site reactions, and the killed ERA-2GnRH vaccine induced high and persistent rabies virus neutralizing antibodies (rVNA) in mice. The rVNA in the hydrogel group reached an average of 327.40 IU/mL, more than 200 times higher than the liquid vaccine alone. The Gonadotropin-releasing hormone (GnRH) antibodies were also present and lasted longer in the hydrogel group, but did not prevent fertility in mice, reflecting a possible threshold level of GnRH antibodies for contraception. In conclusion, the hydrogel facilitated a high and long-lasting immunity, and ERA-2GnRH is a promising dual vaccine candidate. Future studies will focus on rabies protection in target species and improving the anti-GnRH response.
Keywords: ERA-2GnRH; chitosan; gonadotropin-releasing hormone (GnRH); immunocontraceptive vaccine; nonsurgical sterilization; rabies virus; thermo-responsive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest. Use of trade names and commercial sources are for identification only and do not imply endorsement by the U. S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency, or the official position of the Centers for Disease Control and Prevention.
Figures




References
-
- Administration of Rabies Vaccination State Laws. [(accessed on 4 June 2019)]; Available online: https://www.avma.org/Advocacy/ StateAndLocal/Pages/rabies-vaccination.aspx.
-
- Frequently Asked Questions on Rabies. [(accessed on 4 June 2019)]; Available online: https://www.who.int/rabies/resources/ SEA_CD_278_FAQs_Rabies.pdf.
-
- Gibson A.D., Handel I.G., Shervell K., Roux T., Mayer D., Muyila S., Maruwo G.B., Nkhulungo E.M., Foster R.A., Chikungwa P., et al. The Vaccination of 35,000 dogs in 20 working days using combined static point and door-to-door methods in Blantyre, Malawi. PLoS Negl. Trop. Dis. 2016;10:e0004824. doi: 10.1371/journal.pntd.0004824. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

