Impact of HIV-1 Diversity on Its Sensitivity to Neutralization
- PMID: 31349655
- PMCID: PMC6789624
- DOI: 10.3390/vaccines7030074
Impact of HIV-1 Diversity on Its Sensitivity to Neutralization
Abstract
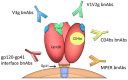
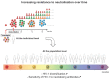
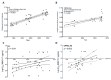
The HIV-1 pandemic remains a major burden on global public health and a vaccine to prevent HIV-1 infection is highly desirable but has not yet been developed. Among the many roadblocks to achieve this goal, the high antigenic diversity of the HIV-1 envelope protein (Env) is one of the most important and challenging to overcome. The recent development of broadly neutralizing antibodies has considerably improved our knowledge on Env structure and its interplay with neutralizing antibodies. This review aims at highlighting how the genetic diversity of HIV-1 thwarts current, and possibly future, vaccine developments. We will focus on the impact of HIV-1 Env diversification on the sensitivity to neutralizing antibodies and the repercussions of this continuous process at a population level.
Keywords: HIV-1 vaccine; broadly neutralizing antibodies; diversity; envelope; evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mascola J.R., Stiegler G., VanCott T.C., Katinger H., Carpenter C.B., Hanson C.E., Beary H., Hayes D., Frankel S.S., Birx D.L., et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. - DOI - PubMed
-
- Shingai M., Nishimura Y., Klein F., Mouquet H., Donau O.K., Plishka R., Buckler-White A., Seaman M., Piatak M., Lifson J.D., et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. - DOI - PMC - PubMed
-
- Julg B., Liu P.-T., Wagh K., Fischer W.M., Abbink P., Mercado N.B., Whitney J.B., Nkolola J.P., McMahan K., Tartaglia L.J., et al. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci. Transl. Med. 2017;9:eaao4235. doi: 10.1126/scitranslmed.aao4235. - DOI - PMC - PubMed
-
- Gilbert P.B., Juraska M., DeCamp A.C., Karuna S., Edupuganti S., Mgodi N., Donnell D.J., Bentley C., Sista N., Andrew P., et al. Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials. Stat. Commun. Infect. Dis. 2017;9:20160001. doi: 10.1515/scid-2016-0001. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

