Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update
- PMID: 31349656
- PMCID: PMC6719186
- DOI: 10.3390/antiox8080244
Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update
Abstract
In recent years, a great deal of attention has been paid to natural compounds due to their many biological effects. Polyphenols are a class of plant derivatives that have been widely investigated for preventing and treating many oxidative stress-related pathological conditions, such as neurodegenerative and cardiovascular diseases, cancer, diabetes mellitus and inflammation. Among these polyphenols, resveratrol (RSV) has attracted considerable interest owing to its high antioxidant and free radical scavenging activities. However, the poor water solubility and rapid metabolism of RSV lead to low bioavailability, thus limiting its clinical efficacy. After discussing the main biochemical mechanisms involved in RSV biological activities, this review will focus on the strategies attempted to improve RSV effectiveness, both for systemic and for topical administration. In particular, technological approaches involving RSV incorporation into different delivery systems such as liposomes, polymeric and lipid nanoparticles, microemulsions and cyclodextrins will be illustrated, highlighting their potential clinical applications. In addition, chemical modifications of this antioxidant aimed at improving its physicochemical properties will be described along with the results of in vitro and in vivo studies.
Keywords: cyclodextrins; drug delivery systems; lipid nanoparticles; liposomes; microemulsions; polymeric nanoparticles; prodrugs; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



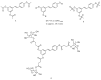
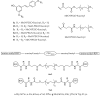
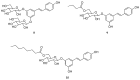



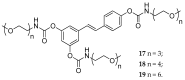



References
-
- Shen T., Xie C.F., Wang X.N., Lou H.X. Stilbenoids. In: Ramawat K., Mérillon J.M., editors. Natural Products. Springer; Berlin, Germany: 2013.
-
- Adrian M., Jeandet P., Veneau J., Weston L.A., Bessis R. Biological Activity of Resveratrol, a Stilbenic Compound from Grapevines, Against Botrytis cinerea, the Causal Agent for Gray Mold. J. Chem. Ecol. 1997;23:1689–1702. doi: 10.1023/B:JOEC.0000006444.79951.75. - DOI
-
- Sobolev V.S., Khan S.I., Tabanca N., Wedge D.E., Manly S.P., Cutler S.J., Coy M.R., Becnel J.J., Neff S.A., Gloer J.B. Biological Activity of Peanut (Arachis hypogaea) Phytoalexins and Selected Natural and Synthetic Stilbenoids. J. Agric. Food Chem. 2011;59:1673–1682. doi: 10.1021/jf104742n. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

