Species of Characidotrema Paperna & Thurston, 1968 (Monogenea: Dactylogyridae) from fishes of the Alestidae (Characiformes) in Africa: new species, host-parasite associations and first insights into the phylogeny of the genus
- PMID: 31349871
- PMCID: PMC6659303
- DOI: 10.1186/s13071-019-3580-y
Species of Characidotrema Paperna & Thurston, 1968 (Monogenea: Dactylogyridae) from fishes of the Alestidae (Characiformes) in Africa: new species, host-parasite associations and first insights into the phylogeny of the genus
Abstract
Background: African tetras (Alestidae) belonging to Brycinus Valenciennes are known to be parasitized with monogeneans attributed to two genera, Annulotrema Paperna & Thurston, 1969 and Characidotrema Paperna & Thurston, 1968 (Dactylogyridae). During a survey of monogeneans parasitizing alestids, species of Characidotrema were collected in Cameroon, D. R. Congo, Senegal, South Africa, Sudan and Zimbabwe. This paper provides new morphological data and the first molecular analysis broadening our knowledge on the diversity of these parasites.
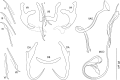
Results: Seven species (four known and three new) of Characidotrema are reported from two species of Brycinus: C. auritum n. sp. and C. vespertilio n. sp. from B. imberi (Peters); and C. brevipenis Paperna, 1969, C. nursei Ergens, 1973, C. pollex n. sp., C. spinivaginus (Paperna, 1973) and C. zelotes Kritsky, Kulo & Boeger, 1987 from B. nurse (Rüppell). Species identification was based on morphological analysis of the sclerotized structures supported by nuclear ribosomal DNA (partial 18S rDNA, ITS1, and 28S rDNA) sequence data. Morphological analysis confirmed that the most apparent character distinguishing species in the genus is the morphology of the male copulatory organ and vagina. Observations on the haptoral sclerotized elements of these parasites by means of phase contrast microscopy revealed the presence of a sheath-like structure relating to the ventral anchor, a feature that supplements the generic diagnosis of Characidotrema. Maximum Likelihood and Bayesian analyses of the large subunit (28S) rDNA sequences recovered Characidotrema species isolated from the two Brycinus hosts as monophyletic, and indicated a closer relationship of this group to monogeneans parasitizing African cyprinids (Dactylogyrus spp.) and cichlids (species of Cichlidogyrus Paperna, 1960, Scutogyrus Pariselle & Euzet, 1995, and Onchobdella Paperna, 1968) than to those from catfishes (species of Quadriacanthus Paperna, 1961, Schilbetrema Paperna & Thurston, 1968 and Synodontella Dossou & Euzet, 1993). The overall agreement between the morphological diversification of the MCOs and the molecular tree observed in this study indicates that significant phylogenetic signals for clarifying relationships among species of Characidotrema are present in the characteristics of the MCO.
Conclusions: It seems that intra-host speciation is an important force shaping the present distribution and diversity of Characidotrema but further studies are necessary to confirm this hypothesis and assess questions related to the phylogeny of these parasites. To identify potential co-speciation events, co-phylogenetic analyses of these monogeneans and their alestid hosts are required.
Keywords: Africa; Alestidae; Brycinus; Characidotrema; DNA; Dactylogyridae; Diversity; Monogenea.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










References
-
- Khalil LF, Polling L. Check list of the helminth parasites of African freshwater fishes. Pietersburg: University of the North; 1997.
-
- Kičinjaová ML, Blažek R, Gelnar M, Řehulková E. Annulotrema (Monogenea: Dactylogyridae) from the gills of African tetras (Characiformes: Alestidae) in Lake Turkana, Kenya, with descriptions of four new species and a redescription of A. elongata Paperna and Thurston, 1969. Parasitol Res. 2015;114:4107–4120. doi: 10.1007/s00436-015-4682-x. - DOI - PubMed
-
- Kritsky DC, Kulo SD, Boeger WA. Resurrection of Characidotrema Paperna and Thurston, 1968 (Monogenea: Dactylogyridae) with the description of two new species from Togo, Africa. Proc Helminthol Soc Wash. 1987;54:175–184.
-
- Paperna I. New species of Monogenea (Vermes) from African freshwater fish. A preliminary report. Rev Zool Bot Afr. 1973;87:505–518.
-
- Paperna I, Thurston JP. Monogenetic trematodes (Dactylogyridae) from fish in Uganda. Rev Zool Bot Afr. 1968;78:284–294.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

