Imaging respiratory muscle quality and function in Duchenne muscular dystrophy
- PMID: 31350642
- PMCID: PMC6810837
- DOI: 10.1007/s00415-019-09481-z
Imaging respiratory muscle quality and function in Duchenne muscular dystrophy
Abstract
Objective: Duchenne muscular dystrophy (DMD) is characterized by damage to muscles including the muscles involved in respiration. Dystrophic muscles become weak and infiltrated with fatty tissue, resulting in progressive respiratory impairment. The objective of this study was to assess respiratory muscle quality and function in DMD using magnetic resonance imaging and to determine the relationship to clinical respiratory function.
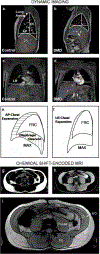
Methods: Individuals with DMD (n = 36) and unaffected controls (n = 12) participated in this cross sectional magnetic resonance imaging study. Participants underwent dynamic imaging of the thorax to assess diaphragm and chest wall mobility and chemical shift-encoded imaging of the chest and abdomen to determine fatty infiltration of the accessory respiratory muscles. Additionally, clinical pulmonary function measures were obtained.
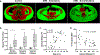
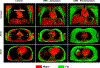
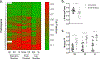
Results: Thoracic cavity area was decreased in individuals with DMD compared to controls during tidal and maximal breathing. Individuals with DMD had reduced chest wall movement in the anterior-posterior direction during maximal inspirations and expirations, but diaphragm descent during maximal inspirations (normalized to height) was only decreased in a subset of individuals with maximal inspiratory pressures less than 60% predicted. Muscle fat fraction was elevated in all three expiratory muscles assessed (p < 0.001), and the degree of fatty infiltration correlated with percent predicted maximal expiratory pressures (r = - 0.70, p < 0.001). The intercostal muscles demonstrated minimal visible fatty infiltration; however, this analysis was qualitative and resolution limited.
Interpretation: This magnetic resonance imaging investigation of diaphragm movement, chest wall movement, and accessory respiratory muscle fatty infiltration provides new insights into the relationship between disease progression and clinical respiratory function.
Keywords: Diaphragm; Dixon imaging; MRI; Neuromuscular disease; Pulmonary.
Conflict of interest statement
Figures

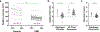
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

