Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro†
- PMID: 31350850
- PMCID: PMC6877782
- DOI: 10.1093/biolre/ioz139
Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro†
Abstract
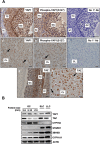
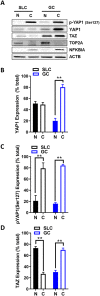
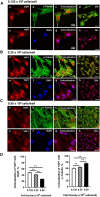
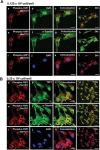
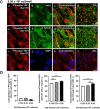
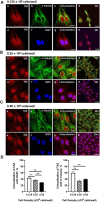
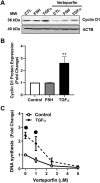
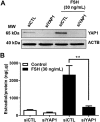
Yes-associated protein 1 (YAP1) is a major component of the Hippo signaling pathway. Although the exact extracellular signals that control the Hippo pathway are currently unknown, increasing evidence supports a critical role for the Hippo pathway in embryonic development, regulation of organ size, and carcinogenesis. Granulosa cells (GCs) within the ovarian follicle proliferate and produce steroids and growth factors, which facilitate the growth of follicle and maturation of the oocyte. We hypothesize that YAP1 plays a role in proliferation and estrogen secretion of GCs. In the current study, we examined the expression of the Hippo signaling pathway in bovine ovaries and determined whether it was important for GC proliferation and estrogen production. Mammalian STE20-like protein kinase 1 (MST1) and large tumor suppressor kinase 2 (LATS2) were identified as prominent upstream components of the Hippo pathway expressed in granulosa and theca cells of the follicle and large and small cells of the corpus luteum. Immunohistochemistry revealed that YAP1 was localized to the nucleus of growing follicles. In vitro, nuclear localization of the downstream Hippo signaling effector proteins YAP1 and transcriptional co-activator with PDZ-binding motif (TAZ) was inversely correlated with GC density, with greater nuclear localization under conditions of low cell density. Treatment with verteporfin and siRNA targeting YAP1 or TAZ revealed a critical role for these transcriptional co-activators in GC proliferation. Furthermore, knockdown of YAP1 in GCs inhibited follicle-stimulating hormone (FSH)-induced estradiol biosynthesis. The data indicate that Hippo pathway transcription co-activators YAP1/TAZ play an important role in GC proliferation and estradiol synthesis, two processes necessary for maintaining normal follicle development.
Keywords: Hippo signaling; Yes-associated protein 1; bovine; follicular development; granulosa cells; proliferation; steroidogenesis.
Published by Oxford University Press on behalf of Society for the Study of Reproduction 2019. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Williams CJ. Erickson GF. Morphology and Physiology of the Ovary. [Updated 2012. Jan 30]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278951/ - PubMed
-
- Kranc W, Budna J, Kahan R, Chachuła A, Bryja A, Ciesiółka S, Borys S, Antosik M, Bukowska D, Brussow K. Molecular basis of growth, proliferation, and differentiation of mammalian follicular granulosa cells. J Biol Regul Homeost Agents 2017; 31:1–8. - PubMed
-
- Fitzpatrick SL, Richards JS. Regulation of the rat aromatase gene in ovarian granulosa cells and R2C Leydig cells. J Steroid Biochem Mol Biol 1993; 44:429–433. - PubMed
-
- Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 1998; 12:924–940v. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

