DAXX in cancer: phenomena, processes, mechanisms and regulation
- PMID: 31350900
- PMCID: PMC6735914
- DOI: 10.1093/nar/gkz634
DAXX in cancer: phenomena, processes, mechanisms and regulation
Abstract
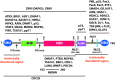
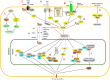
DAXX displays complex biological functions. Remarkably, DAXX overexpression is a common feature in diverse cancers, which correlates with tumorigenesis, disease progression and treatment resistance. Structurally, DAXX is modular with an N-terminal helical bundle, a docking site for many DAXX interactors (e.g. p53 and ATRX). DAXX's central region folds with the H3.3/H4 dimer, providing a H3.3-specific chaperoning function. DAXX has two functionally critical SUMO-interacting motifs. These modules are connected by disordered regions. DAXX's structural features provide a framework for deciphering how DAXX mechanistically imparts its functions and how its activity is regulated. DAXX modulates transcription through binding to transcription factors, epigenetic modifiers, and chromatin remodelers. DAXX's localization in the PML nuclear bodies also plays roles in transcriptional regulation. DAXX-regulated genes are likely important effectors of its biological functions. Deposition of H3.3 and its interactions with epigenetic modifiers are likely key events for DAXX to regulate transcription, DNA repair, and viral infection. Interactions between DAXX and its partners directly impact apoptosis and cell signaling. DAXX's activity is regulated by posttranslational modifications and ubiquitin-dependent degradation. Notably, the tumor suppressor SPOP promotes DAXX degradation in phase-separated droplets. We summarize here our current understanding of DAXX's complex functions with a focus on how it promotes oncogenesis.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Identification of two independent SUMO-interacting motifs in Daxx: evolutionary conservation from Drosophila to humans and their biochemical functions.Cell Cycle. 2009 Jan 1;8(1):76-87. doi: 10.4161/cc.8.1.7493. Cell Cycle. 2009. PMID: 19106612
-
The Dynamic Regulation of Daxx-Mediated Transcriptional Inhibition by SUMO and PML NBs.Int J Mol Sci. 2025 Jul 12;26(14):6703. doi: 10.3390/ijms26146703. Int J Mol Sci. 2025. PMID: 40724953 Free PMC article. Review.
-
E1B-55K-Mediated Regulation of RNF4 SUMO-Targeted Ubiquitin Ligase Promotes Human Adenovirus Gene Expression.J Virol. 2018 Jun 13;92(13):e00164-18. doi: 10.1128/JVI.00164-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29695423 Free PMC article.
-
Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors.Mol Cell. 2006 Nov 3;24(3):341-54. doi: 10.1016/j.molcel.2006.10.019. Mol Cell. 2006. PMID: 17081986
-
[Progress on the subcellular localization of Daxx].Ai Zheng. 2009 Dec;28(12):1333-6. doi: 10.5732/cjc.009.10168. Ai Zheng. 2009. PMID: 19958631 Review. Chinese.
Cited by
-
The SUMO Family: Mechanisms and Implications in Thyroid Cancer Pathogenesis and Therapy.Biomedicines. 2024 Oct 21;12(10):2408. doi: 10.3390/biomedicines12102408. Biomedicines. 2024. PMID: 39457720 Free PMC article. Review.
-
Synergistic cytotoxicity of fludarabine, clofarabine, busulfan, vorinostat and olaparib in AML cells.Front Oncol. 2023 Nov 23;13:1287444. doi: 10.3389/fonc.2023.1287444. eCollection 2023. Front Oncol. 2023. PMID: 38074694 Free PMC article.
-
Daxx maintains endogenous retroviral silencing and restricts cellular plasticity in vivo.Sci Adv. 2020 Aug 5;6(32):eaba8415. doi: 10.1126/sciadv.aba8415. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32821827 Free PMC article.
-
Biomolecular condensates: Formation mechanisms, biological functions, and therapeutic targets.MedComm (2020). 2023 Feb 28;4(2):e223. doi: 10.1002/mco2.223. eCollection 2023 Apr. MedComm (2020). 2023. PMID: 36875159 Free PMC article. Review.
-
Molecular Signature Expands the Landscape of Driver Negative Thyroid Cancers.Cancers (Basel). 2021 Oct 15;13(20):5184. doi: 10.3390/cancers13205184. Cancers (Basel). 2021. PMID: 34680332 Free PMC article.
References
-
- Kress T.R., Sabo A., Amati B.. MYC: connecting selective transcriptional control to global RNA production. Nat. Rev. Cancer. 2015; 15:593–607. - PubMed
-
- Wolpaw A.J., Dang C.V.. MYC-induced metabolic stress and tumorigenesis. Biochim. Biophys. Acta Rev. Cancer. 2018; 1870:43–50. - PubMed
-
- Chang H.Y., Nishitoh H., Yang X., Ichijo H., Baltimore D.. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998; 281:1860–1863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

