Structural and functional study of FK domain of Fstl1
- PMID: 31351024
- PMCID: PMC6739823
- DOI: 10.1002/pro.3696
Structural and functional study of FK domain of Fstl1
Abstract
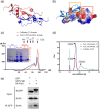
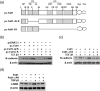
Fstl1 is a TGF-β superfamily binding protein which involved in many pathological processes. The function of Fstl1 has been widely elucidated, but its structural characterization has not been explored. Here we solved the high-resolution crystal structure of FK domain of murine Fstl1, analyzed its unique characteristics, and investigated its contribution to the function of full-length Fstl1. We found that Fstl1-FK forms a stable dimer in both solution and crystal, which suggest that this protein may function as a dimer during its interaction with TGF-β, a molecule known to form dimer during activation process. We also found this FK domain is indispensable for the proper function of Fstl1 during the transduction of TGF-β signaling. These observations provide important insights into the understanding of Fstl1 and may facilitate the exploration of this molecule in clinical study.
Keywords: FK domain; Fstl1; TGF-β signaling; crystal structure; lung fibrosis.
© 2019 The Protein Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice.J Exp Med. 2015 Feb 9;212(2):235-52. doi: 10.1084/jem.20121878. Epub 2015 Jan 12. J Exp Med. 2015. PMID: 25584011 Free PMC article.
-
TGF-β1 induces Fstl1 via the Smad3-c-Jun pathway in lung fibroblasts.Am J Physiol Lung Cell Mol Physiol. 2017 Aug 1;313(2):L240-L251. doi: 10.1152/ajplung.00523.2016. Epub 2017 May 11. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28495857
-
FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2.Gut. 2022 Dec;71(12):2539-2550. doi: 10.1136/gutjnl-2021-325150. Epub 2022 Feb 9. Gut. 2022. PMID: 35140065 Free PMC article.
-
Follistatin-like 1 in vertebrate development.Birth Defects Res C Embryo Today. 2013 Mar;99(1):61-9. doi: 10.1002/bdrc.21030. Birth Defects Res C Embryo Today. 2013. PMID: 23723173 Review.
-
FSTL1: A double-edged sword in cancer development.Gene. 2024 May 15;906:148263. doi: 10.1016/j.gene.2024.148263. Epub 2024 Feb 10. Gene. 2024. PMID: 38346455 Review.
Cited by
-
FSTL1 and TLR4 interact with PEDV structural proteins to promote virus adsorption to host cells.J Virol. 2025 Jan 31;99(1):e0183724. doi: 10.1128/jvi.01837-24. Epub 2024 Dec 13. J Virol. 2025. PMID: 39670742 Free PMC article.
-
The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging.Immunity. 2022 Sep 13;55(9):1609-1626.e7. doi: 10.1016/j.immuni.2022.07.007. Epub 2022 Aug 12. Immunity. 2022. PMID: 35963236 Free PMC article.
-
Follistatin-like 1 promotes proliferation of matured human hypoxic iPSC-cardiomyocytes and is secreted by cardiac fibroblasts.Mol Ther Methods Clin Dev. 2022 Feb 23;25:3-16. doi: 10.1016/j.omtm.2022.02.005. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35317048 Free PMC article.
-
The Expression of Follistatin-like 1 Protein Is Associated with the Activation of the EMT Program in Sjögren's Syndrome.J Clin Med. 2022 Sep 13;11(18):5368. doi: 10.3390/jcm11185368. J Clin Med. 2022. PMID: 36143013 Free PMC article.
-
FSTL1 accelerates nucleus pulposus-derived mesenchymal stem cell apoptosis in intervertebral disc degeneration by activating TGF-β-mediated Smad2/3 phosphorylation.J Transl Med. 2025 Feb 26;23(1):232. doi: 10.1186/s12967-025-06231-w. J Transl Med. 2025. PMID: 40011941 Free PMC article.
References
-
- Sylva M, Moorman AF, van den Hoff MJ. Follistatin‐like 1 in vertebrate development. Birth Defects Res C Embryo Today. 2013;99:61–69. - PubMed
-
- Gagliardi F, Narayanan A, Mortini P. SPARCL1 a novel player in cancer biology. Crit Rev Oncol Hematol. 2017;109:63–68. - PubMed
-
- Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin‐like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

