Structural and functional study of FK domain of Fstl1
- PMID: 31351024
- PMCID: PMC6739823
- DOI: 10.1002/pro.3696
Structural and functional study of FK domain of Fstl1
Abstract
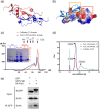
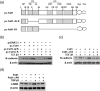
Fstl1 is a TGF-β superfamily binding protein which involved in many pathological processes. The function of Fstl1 has been widely elucidated, but its structural characterization has not been explored. Here we solved the high-resolution crystal structure of FK domain of murine Fstl1, analyzed its unique characteristics, and investigated its contribution to the function of full-length Fstl1. We found that Fstl1-FK forms a stable dimer in both solution and crystal, which suggest that this protein may function as a dimer during its interaction with TGF-β, a molecule known to form dimer during activation process. We also found this FK domain is indispensable for the proper function of Fstl1 during the transduction of TGF-β signaling. These observations provide important insights into the understanding of Fstl1 and may facilitate the exploration of this molecule in clinical study.
Keywords: FK domain; Fstl1; TGF-β signaling; crystal structure; lung fibrosis.
© 2019 The Protein Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Sylva M, Moorman AF, van den Hoff MJ. Follistatin‐like 1 in vertebrate development. Birth Defects Res C Embryo Today. 2013;99:61–69. - PubMed
-
- Gagliardi F, Narayanan A, Mortini P. SPARCL1 a novel player in cancer biology. Crit Rev Oncol Hematol. 2017;109:63–68. - PubMed
-
- Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin‐like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

