Astrocyte-Neuron Interactions in the Striatum: Insights on Identity, Form, and Function
- PMID: 31351745
- PMCID: PMC6741427
- DOI: 10.1016/j.tins.2019.06.003
Astrocyte-Neuron Interactions in the Striatum: Insights on Identity, Form, and Function
Abstract
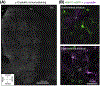
The physiological functions of astrocytes within neural circuits remain incompletely understood. There has been progress in this regard from recent work on striatal astrocytes, where detailed studies are emerging. In this review, findings on striatal astrocyte identity, form, and function, are summarized with a focus on how astrocytes regulate striatal neurons, circuits, and behavior. Specific features of striatal astrocytes are highlighted to illustrate how they may be specialized to regulate medium spiny neurons (MSNs) by responding to, and altering, excitation and inhibition. Further experiments should reveal additional mechanisms for astrocyte-neuron interactions in the striatum and potentially reveal insights into the functions of astrocytes in neural circuits more generally.
Keywords: astrocyte; basal ganglia; behavior; microcircuit; morphology; striatum.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


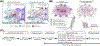

References
-
- Kettenmann H and Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci. 31,653–659 - PubMed
-
- Shepherd GM and Grillner S (2010) Handbook of Brain Microcircuits, Oxford University Press

