Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet
- PMID: 31352001
- PMCID: PMC6815712
- DOI: 10.1053/j.gastro.2019.07.030
Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet
Abstract
Background & aims: Obesity is a risk factor for pancreatic cancer. In mice, a high-fat diet (HFD) and expression of oncogenic KRAS lead to development of invasive pancreatic ductal adenocarcinoma (PDAC) by unknown mechanisms. We investigated how oncogenic KRAS regulates the expression of fibroblast growth factor 21, FGF21, a metabolic regulator that prevents obesity, and the effects of recombinant human FGF21 (rhFGF21) on pancreatic tumorigenesis.
Methods: We performed immunohistochemical analyses of FGF21 levels in human pancreatic tissue arrays, comprising 59 PDAC specimens and 45 nontumor tissues. We also studied mice with tamoxifen-inducible expression of oncogenic KRAS in acinar cells (KrasG12D/+ mice) and fElasCreERT mice (controls). KrasG12D/+ mice were placed on an HFD or regular chow diet (control) and given injections of rhFGF21 or vehicle; pancreata were collected and analyzed by histology, immunoblots, quantitative polymerase chain reaction, and immunohistochemistry. We measured markers of inflammation in the pancreas, liver, and adipose tissue. Activity of RAS was measured based on the amount of bound guanosine triphosphate.
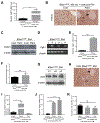
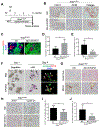
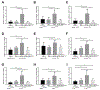
Results: Pancreatic tissues of mice expressed high levels of FGF21 compared with liver tissues. FGF21 and its receptor proteins were expressed by acinar cells. Acinar cells that expressed KrasG12D/+ had significantly lower expression of Fgf21 messenger RNA compared with acinar cells from control mice, partly due to down-regulation of PPARG expression-a transcription factor that activates Fgf21 transcription. Pancreata from KrasG12D/+ mice on a control diet and given injections of rhFGF21 had reduced pancreatic inflammation, infiltration by immune cells, and acinar-to-ductal metaplasia compared with mice given injections of vehicle. HFD-fed KrasG12D/+ mice given injections of vehicle accumulated abdominal fat, developed extensive inflammation, pancreatic cysts, and high-grade pancreatic intraepithelial neoplasias (PanINs); half the mice developed PDAC with liver metastases. HFD-fed KrasG12D/+ mice given injections of rhFGF21 had reduced accumulation of abdominal fat and pancreatic triglycerides, fewer pancreatic cysts, reduced systemic and pancreatic markers of inflammation, fewer PanINs, and longer survival-only approximately 12% of the mice developed PDACs, and none of the mice had metastases. Pancreata from HFD-fed KrasG12D/+ mice given injections of rhFGF21 had lower levels of active RAS than from mice given vehicle.
Conclusions: Normal acinar cells from mice and humans express high levels of FGF21. In mice, acinar expression of oncogenic KRAS significantly reduces FGF21 expression. When these mice are placed on an HFD, they develop extensive inflammation, pancreatic cysts, PanINs, and PDACs, which are reduced by injection of FGF21. FGF21 also reduces the guanosine triphosphate binding capacity of RAS. FGF21 might be used in the prevention or treatment of pancreatic cancer.
Keywords: FGFR1; Gene Regulation; KLB; Signaling.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice.Gastroenterology. 2014 Nov;147(5):1119-33.e4. doi: 10.1053/j.gastro.2014.08.002. Epub 2014 Aug 12. Gastroenterology. 2014. PMID: 25127677
-
miR-802 Suppresses Acinar-to-Ductal Reprogramming During Early Pancreatitis and Pancreatic Carcinogenesis.Gastroenterology. 2022 Jan;162(1):269-284. doi: 10.1053/j.gastro.2021.09.029. Epub 2021 Sep 20. Gastroenterology. 2022. PMID: 34547282
-
FGF21 in obesity and cancer: New insights.Cancer Lett. 2021 Feb 28;499:5-13. doi: 10.1016/j.canlet.2020.11.026. Epub 2020 Nov 29. Cancer Lett. 2021. PMID: 33264641 Free PMC article. Review.
-
Critical role of oncogenic KRAS in pancreatic cancer (Review).Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
Cited by
-
FGFR4 Inhibitor BLU9931 Attenuates Pancreatic Cancer Cell Proliferation and Invasion While Inducing Senescence: Evidence for Senolytic Therapy Potential in Pancreatic Cancer.Cancers (Basel). 2020 Oct 14;12(10):2976. doi: 10.3390/cancers12102976. Cancers (Basel). 2020. PMID: 33066597 Free PMC article.
-
Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour.Int J Mol Sci. 2021 May 24;22(11):5560. doi: 10.3390/ijms22115560. Int J Mol Sci. 2021. PMID: 34074045 Free PMC article. Review.
-
Tumor initiation and early tumorigenesis: molecular mechanisms and interventional targets.Signal Transduct Target Ther. 2024 Jun 19;9(1):149. doi: 10.1038/s41392-024-01848-7. Signal Transduct Target Ther. 2024. PMID: 38890350 Free PMC article. Review.
-
A microRNA checkpoint for Ca2+ signaling and overload in acute pancreatitis.Mol Ther. 2022 Apr 6;30(4):1754-1774. doi: 10.1016/j.ymthe.2022.01.033. Epub 2022 Jan 22. Mol Ther. 2022. PMID: 35077860 Free PMC article.
-
Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress.Cancers (Basel). 2021 Jan 16;13(2):313. doi: 10.3390/cancers13020313. Cancers (Basel). 2021. PMID: 33467038 Free PMC article. Review.
References
-
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. - PubMed
-
- Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007;11:291–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

