Acute lung injury in neonatal rats causes postsynaptic depression in nucleus tractus solitarii second-order neurons
- PMID: 31352011
- PMCID: PMC8408866
- DOI: 10.1016/j.resp.2019.103250
Acute lung injury in neonatal rats causes postsynaptic depression in nucleus tractus solitarii second-order neurons
Abstract
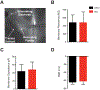
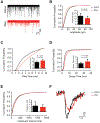
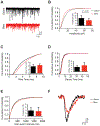
Acute Lung Injury (ALI) alters pulmonary reflex responses, in part due to changes in modulation within the lung and airway neuronal control networks. We hypothesized that synaptic efficacy of nucleus tractus solitarii (nTS) neurons, receiving input from lung, airway, and other viscerosensory afferent fibers, would decrease following ALI. Sprague Dawley neonatal rats (postnatal days 9-11) were given intratracheal installations of saline or bleomycin (a well-characterized model that reproduces the pattern of ALI) and then, one week later, in vitro slices were prepared for whole-cell and perforated whole-cell patch-clamp experiments (postnatal days 16-21). In preparations from ALI rats, 2nd-order nTS neurons had significantly decreased amplitudes of both spontaneous and miniature excitatory postsynaptic currents (sEPSCs and mEPSCs), compared to saline controls. Rise and decay times of sEPSCs were slower in whole-cell recordings from ALI animals. Similarly, the amplitude of tractus solitarii evoked EPSCs (TS-eEPSCs) were significantly lower in 2nd-order nTS neurons from ALI rats. Overall these results suggest the presence of postsynaptic depression at TS-nTS synapses receiving lung, airway, and other viscerosensory afferent tractus solitarii input after bleomycin-induced ALI.
Keywords: Acute lung injury (ALI); Nucleus tractus solitarii (nTS); Synaptic depression; Synaptic efficacy; Tractus solitariievoked excitatory postsynaptic current (TS-eEPSC).
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

