Spatial compartmentalization of lipid droplet biogenesis
- PMID: 31352131
- PMCID: PMC7050823
- DOI: 10.1016/j.bbalip.2019.07.008
Spatial compartmentalization of lipid droplet biogenesis
Abstract
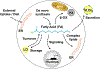
Lipid droplets (LDs) are ubiquitous organelles that store metabolic energy in the form of neutral lipids (typically triacylglycerols and steryl esters). Beyond being inert energy storage compartments, LDs are dynamic organelles that participate in numerous essential metabolic functions. Cells generate LDs de novo from distinct sub-regions at the endoplasmic reticulum (ER), but what determines sites of LD formation remains a key unanswered question. Here, we review the factors that determine LD formation at the ER, and discuss how they work together to spatially and temporally coordinate LD biogenesis. These factors include lipid synthesis enzymes, assembly proteins, and membrane structural requirements. LDs also make contact with other organelles, and these inter-organelle contacts contribute to defining sites of LD production. Finally, we highlight emerging non-canonical roles for LDs in maintaining cellular homeostasis during stress.
Keywords: Endoplasmic reticulum; Fatty acid; Lipid droplet; Lipotoxicity; Metabolon.
Copyright © 2019. Published by Elsevier B.V.
Figures

References
-
- Alvarez HM, and Steinbüchel A (2002). Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60, 367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

