Early medical therapy for acute laryngeal injury (ALgI) following endotracheal intubation: a protocol for a prospective single-centre randomised controlled trial
- PMID: 31352415
- PMCID: PMC6661707
- DOI: 10.1136/bmjopen-2018-027963
Early medical therapy for acute laryngeal injury (ALgI) following endotracheal intubation: a protocol for a prospective single-centre randomised controlled trial
Abstract
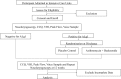
Introduction: Respiratory failure requiring endotracheal intubation accounts for a significant proportion of intensive care unit (ICU) admissions. Little attention has been paid to the laryngeal consequences of endotracheal intubation. Acute laryngeal injury (ALgI) after intubation occurs at the mucosal interface of the endotracheal tube and posterior larynx and although not immediately manifest at extubation, can progress to mature fibrosis, restricted glottic mobility and clinically significant ventilatory impairment. A recent prospective observational study has shown that >50% of patients intubated >24 hours in an ICU develop ALgI. Strikingly, patients with AlgI manifest significantly worse subjective breathing at 12 weeks. Current ALgI treatments are largely surgical yet offer a marginal improvement in symptoms. In this study, we will examine the ability of a postextubation medical regime (azithromycin and inhaled budesonide) to improve breathing 12 weeks after ALgI. METHODS AND ANALYSIS: A prospective, single-centre, double-blinded, randomised, control trial will be conducted at Vanderbilt Medical Center. Participants will be recruited from adult patients in ICUs. Participants will undergo a bedside flexible nasolaryngoscopy for the identification of ALgI within 72 hours postextubation. In addition, participants will be asked to complete peak expiratory flow measurements immediately postintubation. Patients found to have ALgI will be randomised to the placebo control or medical therapy group (azithromycin 250 mg and budesonide 0.5 mg for 14 days). Repeat peak expiratory flow, examination of the larynx and patient-reported Clinical COPD (chronic obstructive pulmonary disease) Questionnaire, Voice Handicap Index and 12-Item Short Form Health Survey questionnaires will be conducted at 12 weeks postextubation. Consented patients will also have patient-specific, disease-specific and procedure-specific covariates abstracted from their medical record.
Ethics and dissemination: The Institutional Review Board (IRB) Committee of the Vanderbilt University Medical Center has approved this protocol (IRB #171066). The findings of the trial will be disseminated through peer-reviewed journals, national and international conferences.
Trial registration number: NCT03250975.
Keywords: acute laryngeal Injury; laryngotracheal stenosis; medical management; post-icu dyspnea.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical