Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation
- PMID: 31353223
- PMCID: PMC6801073
- DOI: 10.1016/j.immuni.2019.06.009
Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation
Abstract
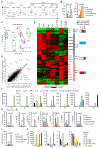
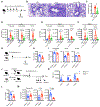
Innate lymphocytes maintain tissue homeostasis at mucosal barriers, with group 2 innate lymphoid cells (ILC2s) producing type 2 cytokines and controlling helminth infection. While the molecular understanding of ILC2 responses has advanced, the complexity of microenvironmental factors impacting ILC2s is becoming increasingly apparent. Herein, we used single-cell analysis to explore the diversity of gene expression among lung lymphocytes during helminth infection. Following infection, we identified a subset of ILC2s that preferentially expressed Il5-encoding interleukin (IL)-5, together with Calca-encoding calcitonin gene-related peptide (CGRP) and its cognate receptor components. CGRP in concert with IL-33 and neuromedin U (NMU) supported IL-5 but constrained IL-13 expression and ILC2 proliferation. Without CGRP signaling, ILC2 responses and worm expulsion were enhanced. Collectively, these data point to CGRP as a context-dependent negative regulatory factor that shapes innate lymphocyte responses to alarmins and neuropeptides during type 2 innate immune responses.
Keywords: CGRP; IL-33; NMU; Nippostrongylus brasiliensis; cytokines; host defense; immunoregulation; innate lymphoid cells; neuropeptides; single-cell RNA-seq.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
D.A. has contributed to scientific advisory boards at MedImmune, Pfizer, FARE and the KRF.
Figures




Comment in
-
The Neuropeptide CGRP Induces Bipolar Syndrome in Group 2 Innate Lymphoid Cells.Immunity. 2019 Oct 15;51(4):598-600. doi: 10.1016/j.immuni.2019.09.015. Immunity. 2019. PMID: 31618650
References
-
- Artis D, and Spits H (2015). The biology of innate lymphoid cells. Nature 517, 293–301. - PubMed
-
- Boudard F, and Bastide M (1991). Inhibition of mouse T-cell proliferation by CGRP and VIP: effects of these neuropeptides on IL-2 production and cAMP synthesis. J Neurosci Res 29, 29–41. - PubMed
-
- Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

