Exploring the best treatment options for BRAF-mutant metastatic colon cancer
- PMID: 31353365
- PMCID: PMC6738120
- DOI: 10.1038/s41416-019-0526-2
Exploring the best treatment options for BRAF-mutant metastatic colon cancer
Abstract
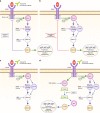
The BRAFV600E mutation is a well-accepted poor prognostic factor in patients with metastatic colorectal cancer (mCRC), as it confers Ras-independent stimulation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway involved in proliferation, migration, angiogenesis and the suppression of apoptosis. Analysis of the potential predictive value of BRAF for treatment efficacy is inherently confounded by this known prognostic impact. Currently, approved therapeutic strategies for patients with BRAF-mutant (BRAF-mt) mCRC are suboptimal, and uncertainty exists regarding how to best treat these patients. Based on the available evidence, it is currently not possible to confirm the superiority of any available treatment options cited in European Society for Medical Oncology and National Comprehensive Cancer Network guidelines (that is, doublet or triplet chemotherapy regimens plus anti-vascular endothelial growth factor or anti-epidermal growth factor receptors), even if triplet chemotherapy plus bevacizumab is the most accepted standard regimen. In this review, we highlight still-emerging strategies that could be deployed to combat BRAF-mt mCRC, including triplet chemotherapy plus available biologic agents, rationally derived combinations of targeted agents and immunotherapy. While it is clear that the needs of patients with BRAF-mt mCRC are currently unmet, we are cautiously optimistic that the recently renewed research interest in these patients will yield clinically relevant insights and therapeutic strategies.
Conflict of interest statement
J.T. declared providing an advisory role for Roche, Merck, KGaA, Amgen Lilly, Baxalta, Servier and Sirtex Medical. A.L.P. declared a consultancy role for MERCK, MERCK SERONO and AMGEN/COHESIA. A.Z. had a consultancy role for Amgen, Baxter, Lilly, Merck Serono, MSD, Roche, Sanofi and Servier. P.L.P. declared a consultancy role for Amgen, Astrazeneca, Biocartis, Boehringer-Ingelheim, Merck, MSD, BMS, Roche and Sanofi.
Figures
Comment in
-
Comment on: "Exploring the best treatment options for BRAF-mutant metastatic colon cancer".Br J Cancer. 2020 May;122(11):1724-1725. doi: 10.1038/s41416-020-0819-5. Epub 2020 Mar 31. Br J Cancer. 2020. PMID: 32231291 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

