Increased Adhesive Potential of Antiphospholipid Syndrome Neutrophils Mediated by β2 Integrin Mac-1
- PMID: 31353826
- PMCID: PMC6935403
- DOI: 10.1002/art.41057
Increased Adhesive Potential of Antiphospholipid Syndrome Neutrophils Mediated by β2 Integrin Mac-1
Abstract
Objective: While the role of antiphospholipid antibodies in activating endothelial cells has been extensively studied, the impact of these antibodies on the adhesive potential of leukocytes has received less attention. This study was undertaken to investigate the extent to which antiphospholipid syndrome (APS) neutrophils adhere to resting endothelial cells under physiologic flow conditions and the surface molecules required for that adhesion.
Methods: Patients with primary APS (n = 43), patients with a history of venous thrombosis but negative test results for antiphospholipid antibodies (n = 11), and healthy controls (n = 38) were studied. Cells were introduced into a flow chamber and perfused across resting human umbilical vein endothelial cells (HUVECs). Surface adhesion molecules were quantified by flow cytometry. Neutrophil extracellular trap release (NETosis) was assessed in neutrophil-HUVEC cocultures.
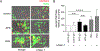
Results: Upon perfusion of anticoagulated blood through the flow chamber, APS neutrophils demonstrated increased adhesion as compared to control neutrophils under conditions representative of either venous (n = 8; P < 0.05) or arterial (n = 15; P < 0.0001) flow. At the same time, APS neutrophils were characterized by up-regulation of CD64, CEACAM1, β2 -glycoprotein I, and activated Mac-1 on their surface (n = 12-18; P < 0.05 for all markers). Exposing control neutrophils to APS plasma or APS IgG resulted in increased neutrophil adhesion (n = 10-11; P < 0.0001) and surface marker up-regulation as compared to controls. A monoclonal antibody specific for activated Mac-1 reduced the adhesion of APS neutrophils in the flow-chamber assay (P < 0.01). The same monoclonal antibody reduced NETosis in neutrophil-HUVEC cocultures (P < 0.01).
Conclusion: APS neutrophils demonstrate increased adhesive potential, which is dependent upon the activated form of Mac-1. In patients, this could lower the threshold for neutrophil-endothelium interactions, NETosis, and possibly thrombotic events.
© 2019, American College of Rheumatology.
Conflict of interest statement
AUTHORSHIP AND CONFLICT OF INTEREST DISCLOSURES
The authors have no competing interests or conflicts to disclose. GS, WJK, KG, SY, APV and ALB conducted experiments and analyzed data. GS, WJK, PLB, OE-A, and JSK designed the study. All authors participated in writing the manuscript, and gave approval before submission.
Figures





References
-
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. - PubMed
-
- Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med. 2018;378(21):2010–21. - PubMed
-
- Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14(5):401–14. - PubMed
-
- Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67(11):2990–3003. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

