TMF inhibits miR-29a/Wnt/β-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes
- PMID: 31354246
- PMCID: PMC6590397
- DOI: 10.2147/DDDT.S209694
TMF inhibits miR-29a/Wnt/β-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes
Retraction in
-
TMF Inhibits miR-29a/Wnt/β-Catenin Signaling Through Upregulating Foxo3a Activity in Osteoarthritis Chondrocytes [Retraction].Drug Des Devel Ther. 2023 Apr 7;17:1063-1064. doi: 10.2147/DDDT.S416064. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37057061 Free PMC article.
Abstract
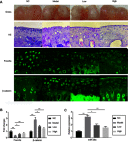
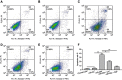
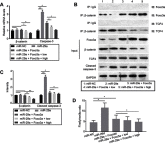
Background: miR-29a, a downstream factor of Wnt/β-catenin signaling, promotes the activity of the Wnt/β-catenin signaling in a positive feedback loop. Our previous work showed that 5,7,3',4'-tetramethoxyflavone (TMF), a major constituent from Murraya exotica L., exhibited chondroprotective activity by inhibiting the activity of Wnt/β-catenin signaling. Purpose: To investigate whether TMF showed the inhibitory effects on miR-29a/β-catenin signaling by up regulation of Foxo3a expression. Methods: Rat knee OA models were duplicated by using Hulth's method. TMF (5 μg/mL and 20 μg/mL) was used for administration to cultured cells, which were isolated from the rat cartilages. Analysis of chondrocytes apoptosis, gene expression, and protein expression were conducted. In addition, miR-29a mimics and pcDNA3.1(+)-Foxo3a vector were used for transfection, luciferase reporter assay for detecting the activity of Wnt/β-catenin signaling, and co-immunoprecipitation for determining proteins interaction. Results: TMF down regulated miR-29a/β-catenin signaling activity and cleaved caspase-3 expression and up regulated Foxo3a expression in OA rat cartilages. In vitro, miR-29a mimics down regulated the expression of Foxo3a and up regulated the activity of Wnt/β-catenin signaling and cleaved caspase-3 expression. TMF ameliorated miR-29a/β-catenin-induced chondrocytes apoptosis by up regulation of Foxo3a expression. Conclusion: TMF exhibited chondroprotective activity by up regulating Foxo3a expression and subsequently inhibiting miR-29a/Wnt/β-catenin signaling activity.
Keywords: Foxo3a; TMF; Wnt/β-catenin; chondrocytes apoptosis; miR-29a; osteoarthritis.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
5,7,3',4'-tetramethoxyflavone ameliorates cholesterol dysregulation by mediating SIRT1/FOXO3a/ABCA1 signaling in osteoarthritis chondrocytes.Future Med Chem. 2021 Dec;13(24):2153-2166. doi: 10.4155/fmc-2021-0247. Epub 2021 Oct 5. Future Med Chem. 2021. PMID: 34608806
-
5,7,3',4'-Tetramethoxyflavone exhibits chondroprotective activity by targeting β-catenin signaling in vivo and in vitro.Biochem Biophys Res Commun. 2014 Sep 26;452(3):682-8. doi: 10.1016/j.bbrc.2014.08.129. Epub 2014 Sep 1. Biochem Biophys Res Commun. 2014. PMID: 25193704
-
MicroRNA-320c inhibits development of osteoarthritis through downregulation of canonical Wnt signaling pathway.Life Sci. 2019 Jul 1;228:242-250. doi: 10.1016/j.lfs.2019.05.011. Epub 2019 May 8. Life Sci. 2019. PMID: 31075235
-
The Interaction between microRNAs and the Wnt/β-Catenin Signaling Pathway in Osteoarthritis.Int J Mol Sci. 2021 Sep 13;22(18):9887. doi: 10.3390/ijms22189887. Int J Mol Sci. 2021. PMID: 34576049 Free PMC article. Review.
-
Progress in the study of FOXO3a interacting with microRNA to regulate tumourigenesis development.Front Oncol. 2023 Oct 27;13:1293968. doi: 10.3389/fonc.2023.1293968. eCollection 2023. Front Oncol. 2023. PMID: 37965449 Free PMC article. Review.
Cited by
-
Proteomics-based network pharmacology and molecular docking reveal the potential mechanisms of 5,6,7,4'-tetramethoxyflavone against HeLa cancer cells.Heliyon. 2024 Oct 4;10(20):e38951. doi: 10.1016/j.heliyon.2024.e38951. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39449708 Free PMC article.
-
Hydroxylated Tetramethoxyflavone Affects Intestinal Cell Permeability and Inhibits Cytochrome P450 Enzymes.Molecules. 2024 Jan 9;29(2):322. doi: 10.3390/molecules29020322. Molecules. 2024. PMID: 38257234 Free PMC article.
-
A comprehensive review of the botany, phytochemistry, pharmacology, and toxicology of Murrayae Folium et Cacumen.Front Pharmacol. 2024 Mar 28;15:1337161. doi: 10.3389/fphar.2024.1337161. eCollection 2024. Front Pharmacol. 2024. PMID: 38606170 Free PMC article. Review.
-
The Emerging Role of Circular RNA Homeodomain Interacting Protein Kinase 3 and Circular RNA 0046367 through Wnt/Beta-Catenin Pathway on the Pathogenesis of Nonalcoholic Steatohepatitis in Egyptian Patients.Rep Biochem Mol Biol. 2023 Jan;11(4):614-625. doi: 10.52547/rbmb.11.4.614. Rep Biochem Mol Biol. 2023. PMID: 37131898 Free PMC article.
-
RNA binding protein PUM2 promotes IL-1β-induced apoptosis of chondrocytes via regulating FOXO3 expression.Heliyon. 2024 Jan 27;10(3):e25080. doi: 10.1016/j.heliyon.2024.e25080. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38356524 Free PMC article.
References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

