Patient and physician preferences for ulcerative colitis treatments in the United States
- PMID: 31354328
- PMCID: PMC6572717
- DOI: 10.2147/CEG.S206970
Patient and physician preferences for ulcerative colitis treatments in the United States
Abstract
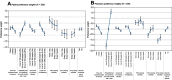
Purpose: This study aimed to elicit patient and physician preferences for ulcerative colitis (UC) treatments in the United States (US). Patients and methods: The following UC treatment attributes included in the discrete-choice experiment (DCE) were identified during qualitative interviews with both patients and physicians: time to symptom improvement, chance of long-term symptom control, risks of serious infection and malignancy, mode and frequency of administration, and need for steroids. The DCE survey instruments were developed and administered to patients and physicians. A random-parameters logit model was used to estimate preference weights and conditional relative importance for these attributes. Results: A total of 200 patients with moderate to severe UC (status determined using self-reported medication history) and 200 gastroenterologists completed the survey. Patients' average age was 42 years; most (59%) were female. Patients considered symptom control 2.5 times as important as time to symptom improvement and 5-year risk of malignancy almost as important as long-term symptom control (relative importance, 0.79 vs 0.96 for long-term symptom control); they preferred oral to subcutaneous or intravenous administration (relative importance, 0.47 vs 0.11 and 0.18, respectively). For physicians, symptom control was the most important attribute and was five times as important as the risk of malignancy. Conclusion: Both patients and physicians considered long-term symptom control the most important attribute relative to others; however, risk of malignancy was of almost-equal importance to patients but not physicians. Differences between patients' and physicians' preferences highlight the need for improved communication about the relevant benefits and risks of different UC treatments to improve therapeutic decision-making.
Keywords: discrete-choice experiments; maximum acceptable risk; patient preference; physician preference; ulcerative colitis.
Conflict of interest statement
Claire Ervin, Kelley Myers, Marco Boeri, and Brett Hauber are employees of RTI Health Solutions, which were paid consultants to Pfizer in connection with the development of this manuscript. Amy Marren, Macro DiBonaventura, and Joseph C Cappelleri are employees and shareholders of Pfizer. Marco Boeri and Brett Hauber received project funding from Pfizer during the conduct of the study. Dr David T Rubin reports grants and personal fees from Abbvie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda, personal fees from Abgenomics, Allergan, Inc., Arena Pharmaceuticals, Biomica, Bristol-Myers Squibb, Dizal Pharmaceuticals, Ferring Pharmaceuticals, Inc., Lilly, Merck & Co., Inc., Medtronic, Napo Pharmaceuticals, Pfizer, and Target PharmaSolutions, Inc., outside the submitted work. The authors report no other conflicts of interest in this work.
Figures



Similar articles
-
Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: Results From a Large Survey Across Seven European Countries Using a Discrete Choice Experiment.Inflamm Bowel Dis. 2024 Dec 5;30(12):2380-2394. doi: 10.1093/ibd/izae015. Inflamm Bowel Dis. 2024. PMID: 38503480 Free PMC article.
-
Patient Preferences for Ulcerative Colitis Treatment in the Middle East Region: A Discrete-Choice Experiment.Gastro Hep Adv. 2023 Oct 10;3(2):190-200. doi: 10.1016/j.gastha.2023.10.002. eCollection 2024. Gastro Hep Adv. 2023. PMID: 39129949 Free PMC article.
-
Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment.Patient. 2012;5(1):33-44. doi: 10.2165/11595390-000000000-00000. Patient. 2012. PMID: 22077619
-
Patient preferences for active ulcerative colitis treatments and fecal microbiota transplantation.Ther Adv Chronic Dis. 2024 Mar 26;15:20406223241239168. doi: 10.1177/20406223241239168. eCollection 2024. Ther Adv Chronic Dis. 2024. PMID: 38544906 Free PMC article.
-
What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution?Patient Prefer Adherence. 2015 Oct 27;9:1517-24. doi: 10.2147/PPA.S87229. eCollection 2015. Patient Prefer Adherence. 2015. PMID: 26604704 Free PMC article. Review.
Cited by
-
Medication Formulation Preference of Mild and Moderate Ulcerative Colitis Patients: a European Survey.Inflamm Intest Dis. 2023 May 12;8(1):41-49. doi: 10.1159/000530139. eCollection 2023 Jun 1. Inflamm Intest Dis. 2023. PMID: 37711959 Free PMC article.
-
Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: Results From a Large Survey Across Seven European Countries Using a Discrete Choice Experiment.Inflamm Bowel Dis. 2024 Dec 5;30(12):2380-2394. doi: 10.1093/ibd/izae015. Inflamm Bowel Dis. 2024. PMID: 38503480 Free PMC article.
-
The Communicating Needs and Features of IBD Experiences (CONFIDE) Study: US and European Patient and Health Care Professional Perceptions of the Experience and Impact of Symptoms of Moderate-to-Severe Ulcerative Colitis.Inflamm Bowel Dis. 2024 Jun 3;30(6):939-949. doi: 10.1093/ibd/izad142. Inflamm Bowel Dis. 2024. PMID: 37603837 Free PMC article.
-
Patients' and gastroenterologists' preferences regarding outcomes and medication attributes in ulcerative colitis.Ann Gastroenterol. 2025 Mar-Apr;38(2):174-181. doi: 10.20524/aog.2025.0944. Epub 2025 Feb 25. Ann Gastroenterol. 2025. PMID: 40124436 Free PMC article.
-
Patient preferences for advanced therapies in ulcerative colitis using conjoint analysis.Intest Res. 2025 Jul;23(3):318-337. doi: 10.5217/ir.2024.00101. Epub 2024 Oct 14. Intest Res. 2025. PMID: 39397286 Free PMC article.
References
-
- NICE. Surveillance report 2017 – crohn’s disease: management (2012) NICE guideline CG152 and Ulcerative colitis: management (2013) NICE guideline CG166. [Updated June 22, 2017]. Available from: https://www.nice.org.uk/guidance/cg166/resources/surveillance-report-201.... Accessed April 23, 2019.
LinkOut - more resources
Full Text Sources

