Modeling Mammalian Commitment to the Neural Lineage Using Embryos and Embryonic Stem Cells
- PMID: 31354503
- PMCID: PMC6637848
- DOI: 10.3389/fphys.2019.00705
Modeling Mammalian Commitment to the Neural Lineage Using Embryos and Embryonic Stem Cells
Abstract
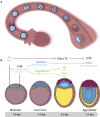
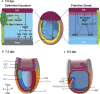
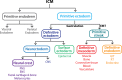
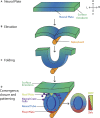
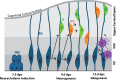
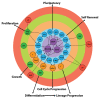
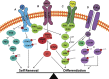
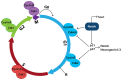
Early mammalian embryogenesis relies on a large range of cellular and molecular mechanisms to guide cell fate. In this highly complex interacting system, molecular circuitry tightly controls emergent properties, including cell differentiation, proliferation, morphology, migration, and communication. These molecular circuits include those responsible for the control of gene and protein expression, as well as metabolism and epigenetics. Due to the complexity of this circuitry and the relative inaccessibility of the mammalian embryo in utero, mammalian neural commitment remains one of the most challenging and poorly understood areas of developmental biology. In order to generate the nervous system, the embryo first produces two pluripotent populations, the inner cell mass and then the primitive ectoderm. The latter is the cellular substrate for gastrulation from which the three multipotent germ layers form. The germ layer definitive ectoderm, in turn, is the substrate for multipotent neurectoderm (neural plate and neural tube) formation, representing the first morphological signs of nervous system development. Subsequent patterning of the neural tube is then responsible for the formation of most of the central and peripheral nervous systems. While a large number of studies have assessed how a competent neurectoderm produces mature neural cells, less is known about the molecular signatures of definitive ectoderm and neurectoderm and the key molecular mechanisms driving their formation. Using pluripotent stem cells as a model, we will discuss the current understanding of how the pluripotent inner cell mass transitions to pluripotent primitive ectoderm and sequentially to the multipotent definitive ectoderm and neurectoderm. We will focus on the integration of cell signaling, gene activation, and epigenetic control that govern these developmental steps, and provide insight into the novel growth factor-like role that specific amino acids, such as L-proline, play in this process.
Keywords: L-proline; amino acids; definitive ectoderm; early primitive ectoderm-like cells; embryonic stem cell; neural; neurectoderm; primitive ectoderm.
Figures
References
-
- Aguilar J., Reyley M. (2005). The uterine tubal fluid: secretion, composition and biological effects. Anim. Reprod. 2, 91–105. Available at: http://www.cbra.org.br/pages/publicacoes/animalreproduction/issues/downl... [Accessed September 10, 2015]
Publication types
LinkOut - more resources
Full Text Sources

