Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus
- PMID: 31354653
- PMCID: PMC6640166
- DOI: 10.3389/fmicb.2019.01544
Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus
Abstract
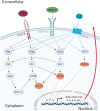
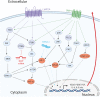
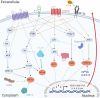
Angiogenesis is the biological process by which new blood vessels are formed from pre-existing vessels. It is considered one of the classic hallmarks of cancer, as pathological angiogenesis provides oxygen and essential nutrients to growing tumors. Two of the seven known human oncoviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), belong to the Gammaherpesvirinae subfamily. Both viruses are associated with several malignancies including lymphomas, nasopharyngeal carcinomas, and Kaposi's sarcoma. The viral genomes code for a plethora of viral factors, including proteins and non-coding RNAs, some of which have been shown to deregulate angiogenic pathways and promote tumor growth. In this review, we discuss the ability of both viruses to modulate the pro-angiogenic process.
Keywords: Epstein–Barr virus; Kaposi’s sarcoma-associated herpesvirus; angiogenesis; gammaherpesviruses; oncoviruses; vascular endothelial growth factor.
Figures

References
-
- An F. Q., Folarin H. M., Compitello N., Roth J., Gerson S. L., McCrae K. R., et al. (2006). Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 80 4833–4846. 10.1128/JVI.80.10.4833-4846.2006 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

