Post-translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions
- PMID: 31354686
- PMCID: PMC6640162
- DOI: 10.3389/fmicb.2019.01604
Post-translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions
Abstract
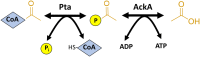
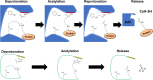
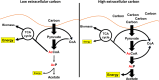
Post-translational modifications (PTM) decorate proteins to provide functional heterogeneity to an existing proteome. The large number of known PTMs highlights the many ways that cells can modify their proteins to respond to diverse stimuli. Recently, PTMs have begun to receive increased interest because new sensitive proteomics workflows and structural methodologies now allow researchers to obtain large-scale, in-depth and unbiased information concerning PTM type and site localization. However, few PTMs have been extensively assessed for functional consequences, leaving a large knowledge gap concerning the inner workings of the cell. Here, we review understanding of N-𝜀-lysine acetylation in bacteria, a PTM that was largely ignored in bacteria until a decade ago. Acetylation is a modification that can dramatically change the function of a protein through alteration of its properties, including hydrophobicity, solubility, and surface properties, all of which may influence protein conformation and interactions with substrates, cofactors and other macromolecules. Most bacteria carry genes predicted to encode the lysine acetyltransferases and lysine deacetylases that add and remove acetylations, respectively. Many bacteria also exhibit acetylation activities that do not depend on an enzyme, but instead on direct transfer of acetyl groups from the central metabolites acetyl coenzyme A or acetyl phosphate. Regardless of mechanism, most central metabolic enzymes possess lysines that are acetylated in a regulated fashion and many of these regulated sites are conserved across the spectrum of bacterial phylogeny. The interconnectedness of acetylation and central metabolism suggests that acetylation may be a response to nutrient availability or the energy status of the cell. However, this and other hypotheses related to acetylation remain untested.
Keywords: acetylation; bacteria; lysine acetyltransferase; mass spectrometry; proteomics.
Figures

References
-
- Amin R., Franz-Wachtel M., Tiffert Y., Heberer M., Meky M., Ahmed Y., et al. (2016). Post-translational serine/threonine phosphorylation and lysine acetylation: a novel regulatory aspect of the global nitrogen response regulator GlnR in S. coelicolor M145. Front. Mol. Biosci. 3:38. 10.3389/fmolb.2016.00038 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

