Neuroinflammation and Myelin Status in Alzheimer's Disease, Parkinson's Disease, and Normal Aging Brains: A Small Sample Study
- PMID: 31354934
- PMCID: PMC6637678
- DOI: 10.1155/2019/7975407
Neuroinflammation and Myelin Status in Alzheimer's Disease, Parkinson's Disease, and Normal Aging Brains: A Small Sample Study
Abstract
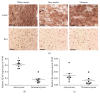
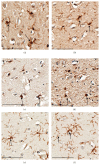
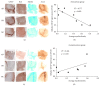
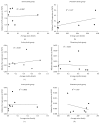
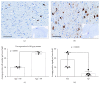
Microglia and astrocytes play important roles in mediating the immune processes and nutritional support in the central nervous system (CNS). Neuroinflammation has been indicated in the progression of neurodegenerative diseases Alzheimer's disease (AD) and Parkinson's disease (PD). Chronic neuroinflammation with sustained activation of microglia and astrocytes may affect white matter tracts and disrupt communication between neurons. Recent studies indicate astrogliosis may inhibit remyelination in demyelinating disorders such as multiple sclerosis. In this study, we investigated the relationship between neuroinflammation and myelin status in postmortem human brain tissue (n = 15 including 6 AD, 5 PD, and 4 age-matched, neurologically normal controls (NC)). We conducted systematic and quantitative immunohistochemistry for glial fibrillary acidic protein (GFAP), ionized calcium-binding adaptor molecule 1 (Iba1), amyloid beta, and highly phosphorylated tau (tauopathy). White matter intactness was evaluated by myelin and axon staining in adjacent brain tissue sections. Eight of 15 cases (4 AD, 3 PD, and 1 NC) showed increased immunoreactivity for microglia and astrocytes in the white matter that connects striatum and cortex. Quantitative analysis of these 8 cases showed a significant negative correlation between GFAP (but not Iba-1) and myelin (but not axon) staining in white matter (r 2 = 0.78, p < 0.005). Tau, but not amyloid beta plaques, is significantly higher in AD vs. PD and NC. Tau burden increases with age in AD cases. These observations indicate that astrocytosis in white matter is associated with loss of myelin in AD, PD, and normal aging and that tau is a potent biomarker for AD.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

