Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study
- PMID: 31355323
- PMCID: PMC6624149
- DOI: 10.1212/NXI.0000000000000590
Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study
Abstract
Objective: To investigate the long-term safety and efficacy of weekly subcutaneous IgPro20 (Hizentra, CSL Behring) in chronic inflammatory demyelinating polyneuropathy (CIDP).
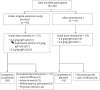
Methods: In a 48-week open-label prospective extension study to the PATH study, patients were initially started on 0.2 g/kg or on 0.4 g/kg weekly and-if clinically stable-switched to 0.2 g/kg weekly after 24 weeks. Upon CIDP relapse on the 0.2 g/kg dose, 0.4 g/kg was (re)initiated. CIDP relapse was defined as a deterioration by at least 1 point in the total adjusted Inflammatory Neuropathy Cause and Treatment score.
Results: Eighty-two patients were enrolled. Sixty-two patients initially received 0.4 g/kg, 20 patients 0.2 g/kg weekly. Seventy-two received both doses during the study. Sixty-six patients (81%) completed the 48-week study duration. Overall relapse rates were 10% in 0.4 g/kg-treated patients and 48% in 0.2 g/kg-treated patients. After dose reduction from 0.4 to 0.2 g/kg, 51% (27/53) of patients relapsed, of whom 92% (24 of 26) improved after reinitiation of the 0.4 g/kg dose. Two-thirds of patients (19/28) who completed the PATH study without relapse remained relapse-free on the 0.2 g/kg dose after dose reduction in the extension study. Sixty-two patients had adverse events (AEs) (76%), of which most were mild or moderate with no related serious AEs.
Conclusions: Subcutaneous treatment with IgPro20 provided long-term benefit at both 0.4 and 0.2 g/kg weekly doses with lower relapse rates on the higher dose. Long-term dosing should be individualized to find the most appropriate dose in a given patient.
Classification of evidence: This study provides Class IV evidence that for patients with CIDP, long-term treatment with SCIG beyond 24 weeks is safe and efficacious.
Figures
References
-
- van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2018;17:35–46. - PubMed
-
- Markvardsen LH, Christiansen I, Andersen H, Jakobsen J. Headache and nausea after treatment with high-dose subcutaneous versus intravenous immunoglobulin. Basic Clin Pharmacol Toxicol 2015;117:409–412. - PubMed
-
- Markvardsen LH, Christiansen I, Harbo T, Jakobsen J. Hemolytic anemia following high dose intravenous immunoglobulin in patients with chronic neurological disorders. Eur J Neurol 2014;21:147–152. - PubMed
-
- Berger M, Rojavin MA, Kiessling P, Zenker O. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol 2011;139:133–141. - PubMed