Exploiting Nanomaterial-mediated Autophagy for Cancer Therapy
- PMID: 31355327
- PMCID: PMC6660170
- DOI: 10.1002/smtd.201800365
Exploiting Nanomaterial-mediated Autophagy for Cancer Therapy
Abstract
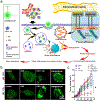
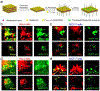
Autophagy is a conserved process that is critical for sequestering and degrading proteins, damaged or aged organelles, and for maintaining cellular homeostasis under stress conditions. Despite its dichotomous role in health and diseases, autophagy usually promotes growth and progression of advanced cancers. In this context, clinical trials using chloroquine and hydroxychloroquine as autophagy inhibitors have suggested that autophagy inhibition is a promising approach for treating advanced malignancies and/or overcoming drug resistance of small molecule therapeutics (i.e., chemotherapy and molecularly targeted therapy). Efficient delivery of autophagy inhibitors may further enhance the therapeutic effect, reduce systemic toxicity, and prevent drug resistance. As such, nanocarriers-based drug delivery systems have several distinct advantages over free autophagy inhibitors that include increased circulation of the drugs, reduced off-target systemic toxicity, increased drug delivery efficiency, and increased solubility and stability of the encapsulated drugs. With their versatile drug encapsulation and surface-functionalization capabilities, nanocarriers can be engineered to deliver autophagy inhibitors to tumor sites in a context-specific and/or tissue-specific manner. This review focuses on the role of nanomaterials utilizing autophagy inhibitors for cancer therapy, with a focus on their applications in different cancer types.
Keywords: Autophagy; cancer; drug delivery; nanomaterials; theranostics.
Conflict of interest statement
Conflicts of interest The authors declare no competing financial interest.
Figures


Similar articles
-
Pro-Death or Pro-Survival: Contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy.Acc Chem Res. 2019 Nov 19;52(11):3164-3176. doi: 10.1021/acs.accounts.9b00397. Epub 2019 Oct 17. Acc Chem Res. 2019. PMID: 31621285 Review.
-
Targeting autophagy in cancer.Cancer. 2018 Aug;124(16):3307-3318. doi: 10.1002/cncr.31335. Epub 2018 Apr 19. Cancer. 2018. PMID: 29671878 Free PMC article. Review.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Targeted regulation of autophagy using nanoparticles: New insight into cancer therapy.Biochim Biophys Acta Mol Basis Dis. 2022 Mar 1;1868(3):166326. doi: 10.1016/j.bbadis.2021.166326. Epub 2021 Dec 20. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 34942307 Review.
-
Application of Nanomaterials and Related Drug Delivery Systems in Autophagy.Molecules. 2024 Jul 26;29(15):3513. doi: 10.3390/molecules29153513. Molecules. 2024. PMID: 39124918 Free PMC article. Review.
Cited by
-
Rationally designed anti-autophagy nanosystems for reversing the immunosuppressive network in the tumor environment.Nanomedicine (Lond). 2025 Jun;20(12):1429-1440. doi: 10.1080/17435889.2025.2508133. Epub 2025 May 22. Nanomedicine (Lond). 2025. PMID: 40401367
-
Reductive damage induced autophagy inhibition for tumor therapy.Nano Res. 2023;16(4):5226-5236. doi: 10.1007/s12274-022-5139-z. Epub 2022 Nov 22. Nano Res. 2023. PMID: 36465522 Free PMC article.
-
New Advances in Nano-Drug Delivery Systems: Helicobacter pylori and Gastric Cancer.Front Oncol. 2022 May 10;12:834934. doi: 10.3389/fonc.2022.834934. eCollection 2022. Front Oncol. 2022. PMID: 35619913 Free PMC article. Review.
-
Chiral nanomaterials for tumor therapy: autophagy, apoptosis, and photothermal ablation.J Nanobiotechnology. 2021 Jul 22;19(1):220. doi: 10.1186/s12951-021-00965-7. J Nanobiotechnology. 2021. PMID: 34294083 Free PMC article. Review.
-
The impact of nanomaterials on autophagy across health and disease conditions.Cell Mol Life Sci. 2024 Apr 17;81(1):184. doi: 10.1007/s00018-024-05199-y. Cell Mol Life Sci. 2024. PMID: 38630152 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

