Clockophagy is a novel selective autophagy process favoring ferroptosis
- PMID: 31355331
- PMCID: PMC6656546
- DOI: 10.1126/sciadv.aaw2238
Clockophagy is a novel selective autophagy process favoring ferroptosis
Abstract
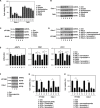
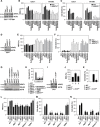
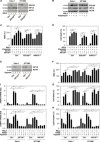
Ferroptosis is a form of nonapoptotic regulated cell death driven by iron-dependent lipid peroxidation. Autophagy involves a lysosomal degradation pathway that can either promote or impede cell death. A high level of autophagy has been associated with ferroptosis, but the mechanisms underpinning this relationship are largely elusive. We characterize the contribution of autophagy to ferroptosis in human cancer cell lines and mouse tumor models. We show that "clockophagy," the selective degradation of the core circadian clock protein ARNTL by autophagy, is critical for ferroptosis. We identify SQSTM1 as a cargo receptor responsible for autophagic ARNTL degradation. ARNTL inhibits ferroptosis by repressing the transcription of Egln2, thus activating the prosurvival transcription factor HIF1A. Genetic or pharmacological interventions blocking ARNTL degradation or inhibiting EGLN2 activation diminished, whereas destabilizing HIF1A facilitated, ferroptotic tumor cell death. Thus, our findings reveal a new pathway, initiated by the autophagic removal of ARNTL, that facilitates ferroptosis induction.
Figures



Comment in
-
Autophagic degradation of the circadian clock regulator promotes ferroptosis.Autophagy. 2019 Nov;15(11):2033-2035. doi: 10.1080/15548627.2019.1659623. Epub 2019 Aug 26. Autophagy. 2019. PMID: 31441366 Free PMC article.
References
-
- Yang W. S., SriRamaratnam R., Welsch M. E., Shimada K., Skouta R., Viswanathan V. S., Cheah J. H., Clemons P. A., Shamji A. F., Clish C. B., Brown L. M., Girotti A. W., Cornish V. W., Schreiber S. L., Stockwell B. R., Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014). - PMC - PubMed
-
- Ran Q., Van Remmen H., Gu M., Qi W., Roberts L. J. II, Prolla T., Richardson A., Embryonic fibroblasts from Gpx4+/− mice: A novel model for studying the role of membrane peroxidation in biological processes. Free Radic. Biol. Med. 35, 1101–1109 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

