Bioinspired mechanically active adhesive dressings to accelerate wound closure
- PMID: 31355332
- PMCID: PMC6656537
- DOI: 10.1126/sciadv.aaw3963
Bioinspired mechanically active adhesive dressings to accelerate wound closure
Abstract
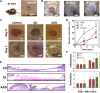
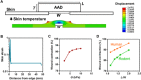
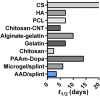
Inspired by embryonic wound closure, we present mechanically active dressings to accelerate wound healing. Conventional dressings passively aid healing by maintaining moisture at wound sites. Recent developments have focused on drug and cell delivery to drive a healing process, but these methods are often complicated by drug side effects, sophisticated fabrication, and high cost. Here, we present novel active adhesive dressings consisting of thermoresponsive tough adhesive hydrogels that combine high stretchability, toughness, tissue adhesion, and antimicrobial function. They adhere strongly to the skin and actively contract wounds, in response to exposure to the skin temperature. In vitro and in vivo studies demonstrate their efficacy in accelerating and supporting skin wound healing. Finite element models validate and refine the wound contraction process enabled by these active adhesive dressings. This mechanobiological approach opens new avenues for wound management and may find broad utility in applications ranging from regenerative medicine to soft robotics.
Figures



References
-
- Singer A. J., Clark R. A. F., Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 (1999). - PubMed
-
- Dyson M., Young S., Pendle C. L., Webster D. F., Lang S. M., Comparison of the effects of moist and dry conditions on dermal repair. J. Invest. Dermatol. 91, 434–439 (1988). - PubMed
-
- Yannas I. V., Burke J. F., Orgill D. P., Skrabut E. M., Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215, 174–176 (1982). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

