STING pathway agonism as a cancer therapeutic
- PMID: 31355488
- PMCID: PMC6814203
- DOI: 10.1111/imr.12765
STING pathway agonism as a cancer therapeutic
Abstract
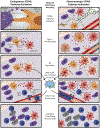
The fact that a subset of human cancers showed evidence for a spontaneous adaptive immune response as reflected by the T cell-inflamed tumor microenvironment phenotype led to the search for candidate innate immune pathways that might be driving such endogenous responses. Preclinical studies indicated a major role for the host STING pathway, a cytosolic DNA sensing pathway, as a proximal event required for optimal type I interferon production, dendritic cell activation, and priming of CD8+ T cells against tumor-associated antigens. STING agonists are therefore being developed as a novel cancer therapeutic, and a greater understanding of STING pathway regulation is leading to a broadened list of candidate immune regulatory targets. Early phase clinical trials of intratumoral STING agonists are already showing promise, alone and in combination with checkpoint blockade. Further advancement will derive from a deeper understanding of STING pathway biology as well as mechanisms of response vs resistance in individual cancer patients.
Keywords: STimulator of INterferon Genes; cancer immunotherapy; innate immunity; tumor immunity; type I interferon.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement:
BAF, EFH, and SL have no known conflicts of interest to report that might influence our objectivity regarding this work. TFG has received consultancy fees from Merck, Roche-Genentech, Abbvie, Bayer, Jounce, Aduro, Fog Pharma, Adaptimmune, FivePrime, Sanofi; Research support from Roche-Genentech, BMS, Merck, Incyte, Seattle Genetics, Celldex, Ono, Evelo, Bayer, Aduro; Intellectual property/licensing agreements with Aduro, Evelo, and BMS; Co-founder/shareholder with Jounce. JJL: Data and Safety Monitoring Board: TTC Oncology; Scientific Advisory Board: 7 Hills, Actym, Alphamab Oncology, Array, BeneVir, Mavu, Pyxis, Tempest Consultancy: Abbvie, Aduro, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Castle, CheckMate, Compugen, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Leap, Merck, Mersana, NewLink, Novartis, RefleXion, Spring Bank, Tempest, Vividio; Research Support: (all to institution for clinical trials unless noted) AbbVie, Array (Scientific Research Agreement; SRA), Boston Biomedical, Bristol-Myers Squibb, Celldex, CheckMate (SRA), Compugen, Corvus, EMD Serono, Evelo (SRA), Delcath, Five Prime, FLX Bio, Genentech, Immunocore, Incyte, Leap, MedImmune, Macrogenics, Novartis, Pharmacyclics, Palleon (SRA), Merck, Tesaro, Xencor; Travel: Array, AstraZeneca, Bayer, BeneVir, Bristol-Myers Squibb, Castle, CheckMate, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Merck, Mersana, NewLink, Novartis, RefleXion; Patents: (both provisional) Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

