IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes
- PMID: 31355779
- PMCID: PMC6715373
- DOI: 10.1172/JCI126363
IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes
Abstract
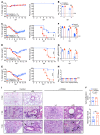
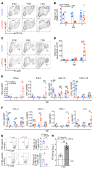
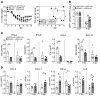
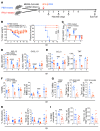
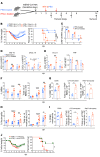
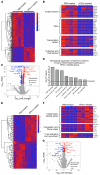
Type 1 IFNs (IFN-I) generally protect mammalian hosts from virus infections, but in some cases, IFN-I is pathogenic. Because IFN-I is protective, it is commonly used to treat virus infections for which no specific approved drug or vaccine is available. The Middle East respiratory syndrome-coronavirus (MERS-CoV) is such an infection, yet little is known about the role of IFN-I in this setting. Here, we show that IFN-I signaling is protective during MERS-CoV infection. Blocking IFN-I signaling resulted in delayed virus clearance, enhanced neutrophil infiltration, and impaired MERS-CoV-specific T cell responses. Notably, IFN-I administration within 1 day after infection (before virus titers peak) protected mice from lethal infection, despite a decrease in IFN-stimulated gene (ISG) and inflammatory cytokine gene expression. In contrast, delayed IFN-β treatment failed to effectively inhibit virus replication, increased infiltration and activation of monocytes, macrophages, and neutrophils in the lungs, and enhanced proinflammatory cytokine expression, resulting in fatal pneumonia in an otherwise sublethal infection. Together, these results suggest that the relative timing of the IFN-I response and maximal virus replication is key in determining outcomes, at least in infected mice. By extension, IFN-αβ or combination therapy may need to be used cautiously to treat viral infections in clinical settings.
Keywords: Infectious disease; Innate immunity; Monocytes; Mouse models; Virology.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

