Epidemiology of Clostridioides difficile infection in Canada: A six-year review to support vaccine decision-making
- PMID: 31355824
- PMCID: PMC6615439
- DOI: 10.14745/ccdr.v45i78a04
Epidemiology of Clostridioides difficile infection in Canada: A six-year review to support vaccine decision-making
Abstract
Background: Two vaccines against Clostridioides difficile infections (CDI) are currently in phase III trials. To enable decision-making on their use in public health programs, national disease epidemiology is necessary.
Objectives: To determine the epidemiology of hospital-acquired CDI (HA-CDI) and community-associated CDI (CA-CDI) in Canada using provincial surveillance data and document discrepancies in CDI-related definitions among provincial surveillance programs.
Methods: Publicly-available CDI provincial surveillance data from 2011 to 2016 that distinguished between HA-CDI and CA-CDI were included and the most common surveillance definitions for each province were used. The HA-, CA-CDI incidence rates and CA-CDI proportions (%) were calculated for each province. Both HA- and CA-CDI incidence rates were examined for trends. Types of disparities were summarized and detailed discrepancies were documented.
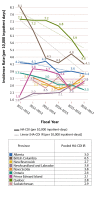
Results: Canadian data were analyzed from nine provinces. The HA-CDI rates ranged from 2.1/10,000 to 6.5/10,000 inpatient-days, with a decreasing trend over time. Available data on CA-CDI showed that both rates and proportions have been increasing over time. Discrepancies among provincial surveillance definitions were documented in CDI case classifications, surveillance populations and rate calculations.
Conclusion: In Canada overall, the rate of HA-CDI has been decreasing and the rate of CA-CDI has been increasing, although this calculation was impeded by discrepancies in CDI-related definitions among provincial surveillance programs. Nationally-adopted common definitions for CDI would enable better comparisons of CDI rates between provinces and a calculation of the pan-Canadian burden of illness to support vaccine decision-making.
Keywords: C. difficile; burden of illness; definitions; epidemiology; surveillance; vaccine.
Conflict of interest statement
Conflict of interest: Y Xia has no conflict of interest MC Tunis, K Amaratunga and A House are employees of the Public Health Agency of Canada C Frenette and K Katz are co-Chairs of CNISP SR Rose is a Past-President of Institute of Public Administration of Canada C Quach is the current Chair of the National Advisory Committee on Immunization and the Past-President for the Association for Medical Microbiology and Infectious Diseases Canada
Figures





References
Footnotes
-
- Alberta Health Services Infection Prevention and Control. Clostridium difficile Infection (CDI) Provincial Surveillance Protocol. AHS 2018. www.albertahealthservices.ca/assets/healthinfo/ipc/hi-ipc-sr-cdi-protoco...
-
- British Columbia Provincial Health Services Authority, Provincial Infection Control Network. Surveillance Protocol for Clostridium difficile Infections (CDI) in BC Acute Care Facilities. PICNet 2017. www.picnet.ca/wp-content/uploads/PICNet-surveillance-protocol-for-CDI-20...
-
- Manitoba, Communicable Disease Control Unit. Clostridium difficile-Associated Diseases (CDAD). 2006.
-
- Brunswick N. Quarterly Hospital Associated Surveillance Report. Quarter 4 (FY 2015/16). www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/CDC/2015-2016_Q4_HAI_...
-
- Labrador N. Provincial Infection Control. Provincial Surveillance Protocol for Clostridium difficile infection. PIC-NL 2013. www.health.gov.nl.ca/health/publichealth/cdc/CDI_surveillance_protocol_f...
References
-
- Canadian Nosocomial Infection Surveillance program (CNISP). Surveillance for Clostridium difficile infection (CDI) Ottawa (ON): PHAC 2018. https://ipac-canada.org/photos/custom/Members/CNISPpublications/CNISP%20...
-
- Public Health Agency of Canada. 2018 Surveillance for Clostridium difficile infection (CDI). PHAC 2017. https://www.ammi.ca/Guideline/44.ENG.pdf
-
- Pan-Canadian Public Health Network; The Communicable and Infectious Disease Steering Committee Antimicrobial Resistance Surveillance Task Group. Antimicrobial Resistance Surveillance Data Requirements for Priority Organisms. PCPHN 2016. www.phn-rsp.ca/pubs/arsdrpo-dsecrao/index-eng.php#3.1
-
- Public Health Agency of Canada. Summary Report on Antimicrobial Resistant Organism (ARO) Surveillance Data from January 1, 2012 to December 31, 2016. Ottawa (ON): PHAC 2018. www.canada.ca/en/public-health/services/publications/science-research-da...