A secreted WY-domain-containing protein present in European isolates of the oomycete Plasmopara viticola induces cell death in grapevine and tobacco species
- PMID: 31356604
- PMCID: PMC6663016
- DOI: 10.1371/journal.pone.0220184
A secreted WY-domain-containing protein present in European isolates of the oomycete Plasmopara viticola induces cell death in grapevine and tobacco species
Abstract
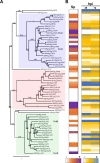
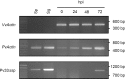
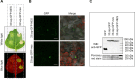
Plasmopara viticola is a biotrophic oomycete pathogen causing grapevine downy mildew. We characterized the repertoire of P. viticola effector proteins which may be translocated into plants to support the disease. We found several secreted proteins that contain canonical dEER motifs and conserved WY-domains but lack the characteristic RXLR motif reported previously from oomycete effectors. We cloned four candidates and showed that one of them, Pv33, induces plant cell death in grapevine and Nicotiana species. This activity is dependent on the nuclear localization of the protein. Sequence similar effectors were present in seven European, but in none of the tested American isolates. Together our work contributes a new type of conserved P. viticola effector candidates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Henry G, Thonart P, Ongena M. PAMPs, MAMPs, DAMPs and others: An update on the diversity of plant immunity elicitors. Biotechnol Agron Soc Environ. 2012; 16: 257–268.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

