Replication and generalization in applied neuroimaging
- PMID: 31356879
- PMCID: PMC6819246
- DOI: 10.1016/j.neuroimage.2019.116048
Replication and generalization in applied neuroimaging
Abstract
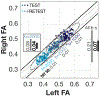
There is much interest in translating neuroimaging findings into meaningful clinical diagnostics. The goal of scientific discoveries differs from clinical diagnostics. Scientific discoveries must replicate under a specific set of conditions; to translate to the clinic we must show that findings using purpose-built scientific instruments will be observable in clinical populations and instruments. Here we describe and evaluate data and computational methods designed to translate a scientific observation to a clinical setting. Using diffusion weighted imaging (DWI), Wahl et al. (2010) observed that across subjects the mean fractional anisotropy (FA) of homologous pairs of tracts is highly correlated. We hypothesize that this is a fundamental biological trait that should be present in most healthy participants, and deviations from this assessment may be a useful diagnostic metric. Using this metric as an illustration of our methods, we analyzed six pairs of homologous white matter tracts in nine different DWI datasets with 44 subjects each. Considering the original FA measurement as a baseline, we show that the new metric is between 2 and 4 times more precise when used in a clinical context. Our framework to translate research findings into clinical practice can be applied, in principle, to other neuroimaging results.
Keywords: Biomarker; Computational reproducibility; DWI; Generalizability; Generalization; Replication; Structural MRI; White matter tracts.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing financial interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Brian Wandell is a co-founder of
Figures


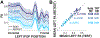



References
-
- Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, Kugel H, Engelen J, Dietsche B, Krug A, Kircher T, Fieremans E, Veraart J, Novikov DS, Boedhoe PSW, van der Werf YD, van den Heuvel OA, Ipser J, Uhlmann A, Stein DJ, Dickie E, Voineskos AN, Malhotra AK, Pizzagalli F, Calhoun VD, Waller L, Veer IM, Walter H, Buchanan RW, Glahn DC, Hong LE, Thompson PM, Kochunov P, 2018. A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain Imaging Behav. 10.1007/s11682-018-9941-x - DOI - PMC - PubMed
-
- Andersson JLR, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888. - PubMed

