A Phase II Study of Ibrutinib in Advanced Neuroendocrine Neoplasms
- PMID: 31357193
- PMCID: PMC7771542
- DOI: 10.1159/000502383
A Phase II Study of Ibrutinib in Advanced Neuroendocrine Neoplasms
Abstract
Background: Ibrutinib is an orally administered inhibitor of Bruton's tyrosine kinase (Btk). Preclinical data suggest that mast cells are recruited within neuroendocrine neoplasms (NENs) where they stimulate angiogenesis and tumor growth. Ibrutinib inhibits mast cell degranulation and has been associated with regression of tumors in a mouse insulinoma model.
Methods: A prospective, phase II trial evaluated patients with advanced gastrointestinal (GI)/lung NENs and pancreatic NENs (pNENs) who had evidence of progression within 12 months of study entry on at least one prior therapy. Patients received ibrutinib 560 mg daily until unacceptable toxicity, progression of disease, or withdrawal of consent. The primary endpoint was objective response rate.
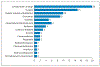
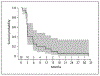
Results: Twenty patients were enrolled on protocol from November 2015 to December 2017 (15 advanced GI/lung NENs and 5 pNENs). No patient reached an objective response. Median PFS was 3.0 months. A total of 44 drug-related adverse events (AEs) were captured as probably or definitely associated with ibrutinib. Five patients experienced probably or definitely related grade 3 AEs, and 1 patient experienced a probably related grade 4 AE. Five patients discontinued treatment prior to radiographic assessment.
Conclusions: Ibrutinib does not show significant evidence of activity in well-differentiated gastroenteropancreatic and lung NENs.
Keywords: Carcinoid tumor; Ibrutinib; Neuroendocrine neoplasm; Pancreatic neuroendocrine tumor.
© 2019 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
Dr. Jonathan R. Strosberg has consulted for Novartis and has received honoraria from Ipsen and Lexicon. Dr. Heloisa P. Soares has received honoraria from Novartis, Ipsen, and Lexicon. None of the other authors declares a personal or financial conflict of interest which could affect the outcome of this study.
Figures
References
-
- Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018. November;68(6):471–87. - PubMed
-
- Cives M, Ghayouri M, Morse B, Brelsford M, Black M, Rizzo A, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016. September;23(9):759–67. - PubMed
-
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007. October;13(10):1211–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical