From Targeting Somatic Mutations to Finding Inherited Cancer Predispositions: The Other Side of the Coin
- PMID: 31357515
- PMCID: PMC6787697
- DOI: 10.3390/diagnostics9030083
From Targeting Somatic Mutations to Finding Inherited Cancer Predispositions: The Other Side of the Coin
Abstract
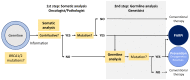
The expanding use of tumor genome analysis by next generation sequencing to drive target therapies has led to increased germline findings in genes predisposing to hereditary cancer. These putative germline findings obtained from theranostic analyses, such as BRCA1/2 gene testing, large panels, whole-exome, or whole-genome sequencing, need to be managed carefully and in an anticipated way with the patient. Before the genetic analysis of a tumor, specific information should be given to patients, who should be aware that the results may have extra-therapeutic medical issues for themselves and relatives. We previously published a list of 36 actionable genes predisposing to cancer for which informing the patient is recommended prior to pangenomic germline analysis because of available screening or preventive strategies. Here, we report clinical practice considerations and schemes for managing germline findings in tumor analyses, including written informed consent and a multidisciplinary approach involving an oncologist, molecular biologist/pathologist, and geneticist in case of germline findings. A somatic result showing a deleterious mutation in a known predisposing gene in a patient who has consented to this purpose should result in referral to a geneticist who is part of the multidisciplinary team. At any time of the somatic analysis process, the patient may have access to a geneticist consultation if additional information is required. This framework will optimally manage both personalized theranostic issues and specific preventive strategies for individuals and relatives; it will also simplify and accelerate the process of genetic testing.
Keywords: cancer predisposing genes; secondary findings; somatic analysis.
Conflict of interest statement
Pr Pujol discloses attending Advisory Board Membership for AstraZeneca, Pfizer, and Roche and research grants from Novartis, Pfizer and Astrazeneca. Dr De La Motte Rouge discloses interests with Roche/Genentech, Novartis, Pfizer, EISAI, Sanofi, AstraZeneca, Tesaro, Clovis Oncology. Pr Penault-LLorca has received consulting fees from Roche, AstraZeneca, Merck Sharp and Dohme, Bristol-Myers Squibb, Novartis, Pfizer, and Sanofi and research grants from Roche and AstraZeneca. The authors declare no support of industries for this work.
Figures

References
-
- Dumbrava E.E.I., Brusco L., Daniels M.S., Wathoo C., Shaw K.R., Lu K.H., Zheng X., Strong L.C., Litton J.K., Arun B., et al. Prevalence of incidental germline pathogenic (PV) and likely pathogenic (LPV) variants in hereditary cancer-related genes identified in matched tumor/normal sequencing of advanced solid tumors. J. Clin. Oncol. 2017;35:1524. doi: 10.1200/JCO.2017.35.15_suppl.1524. - DOI
-
- Abida W., Armenia J., Gopalan A., Brennan R., Walsh M., Barron D., Danila D., Rathkopf D., Morris M., Slovin S., et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00029. - DOI - PMC - PubMed
-
- Pujol P., Perre P.V., Faivre L., Sanlaville D., Corsini C., Baertschi B., Anahory M., Vaur D., Olschwang S., Soufir N., et al. Guidelines for reporting secondary findings of genome sequencing in cancer genes: The SFMPP recommendations. Eur. J. Hum. Genet. 2018;26:1732–1742. doi: 10.1038/s41431-018-0224-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

