Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution
- PMID: 31357662
- PMCID: PMC6723968
- DOI: 10.3390/nu11081741
Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution
Abstract
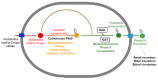
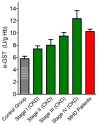
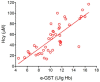
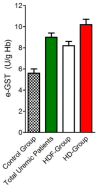
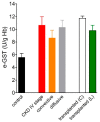
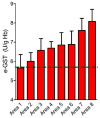
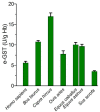
Glutathione transferase P1-1 (GSTP1-1) is expressed in some human tissues and is abundant in mammalian erythrocytes (here termed e-GST). This enzyme is able to detoxify the cell from endogenous and exogenous toxic compounds by using glutathione (GSH) or by acting as a ligandin. This review collects studies that propose GSTP1-1 as a useful biomarker in different fields of application. The most relevant studies are focused on GSTP1-1 as a biosensor to detect blood toxicity in patients affected by kidney diseases. In fact, this detoxifying enzyme is over-expressed in erythrocytes when unusual amounts of toxins are present in the body. Here we review articles concerning the level of GST in chronic kidney disease patients, in maintenance hemodialysis patients and to assess dialysis adequacy. GST is also over-expressed in autoimmune disease like scleroderma, and in kidney transplant patients and it may be used to check the efficiency of transplanted kidneys. The involvement of GSTP in the oxidative stress and in other human pathologies like cancer, liver and neurodegenerative diseases, and psychiatric disorders is also reported. Promising applications of e-GST discussed in the present review are its use for monitoring human subjects living in polluted areas and mammals for veterinary purpose.
Keywords: biomarker; cancer; chronic kidney disease; environmental pollution; glutathione; glutathione transferase; hemodialysis; kidney transplantation; liver disease; neurodegenerative disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
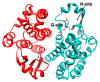




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

