Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer
- PMID: 31357748
- PMCID: PMC6721419
- DOI: 10.3390/cancers11081068
Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer
Abstract
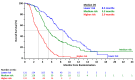
NAPOLI-1 (NCT01494506) was a phase III study of liposomal irinotecan (nal-IRI) plus 5-fluorouracil/leucovorin (5-FU/LV) in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) previously treated with gemcitabine-based therapy. This post hoc analysis of NAPOLI-1 aimed to develop a predictive nomogram for overall survival (OS) at 6 and 12 months. Analyses were derived from all patients in NAPOLI-1 randomized to receive nal-IRI+5-FU/LV, nal-IRI monotherapy, or 5-FU/LV combination therapy. OS was associated with baseline factors using univariate and multivariable Cox analyses. A predictive nomogram was derived and validated using a concordance index and calibration plots. The univariate analyses identified 21 independent factors that contributed to OS, with eight factors significantly associated with OS. The Karnofsky Performance Score contributed the largest number of points (100), followed by presence of liver metastasis (98) and randomization to nal-IRI+5-FU/LV (96). The other baseline factors showing effects were albumin (g/dL), neutrophil/lymphocyte ratio, carbohydrate antigen 19-9 (U/mL), disease stage at diagnosis, and body mass index (kg/m2). The nomogram was used to predict the 6- and 12-month survival probability. The mean absolute errors between the observed and predicted probabilities for OS at 3, 6, and 9 months were 0.07, 0.08, and 0.07, respectively. This nomogram, based on NAPOLI-1, provides additional insight to aid decision-making for patients with mPDAC after previous gemcitabine-based therapy.
Keywords: NAPOLI-1; liposomal irinotecan; nomogram; pancreatic cancer; survival outcomes.
Conflict of interest statement
L.-T.C.: Consulting/Advisory Role: Bristol-Myers Squibb; Five Prime Therapeutics; Lilly; Merrimack; MSD; Novartis; Ono Pharmaceutical; PharmaEngine; Syncope, Taiwan; TTY Biopharm. Research Funding: GlaxoSmithKline (Inst); Merck Serono (Inst); Novartis (Inst); Polaris (Inst); TTY Biopharm (Inst). Patents, Royalties, Other Intellectual Property: anti-alpha-enolase (ENO-1) monoclonal antibody to HuniLife Technology, Taiwan. T.M.: Nothing to disclose. J.-F.B.: Honoraria: Baxalta/Shire; Bayer Schering Pharma; Gilead Sciences. Consulting/Advisory Role: Baxalta/Shire; Bristol-Myers Squibb; Novartis; Onxeo. Travel, Accommodations, Expenses: Bayer Schering Pharma. B.M.: Employed at Ipsen; Stock and Other Ownership Interests with Bristol-Myers Squibb. F.A.d.J.: Employed at Servier; Stock and Other Ownership Interests with Shire. B.B.: Employed at Ipsen and Merrimack. T.B.-S.: Consulting/Advisory Role: AbbVie. Other Relationship (DSMB): Exelixis; Silajen, Armo and Merck. J.T.S.: Consulting/Advisory Role: Baxalta; Celgene; Lilly; Shire. Research Funding: 4SC; Bristol-Myers Squibb; Celgene. Travel, Accommodations, Expenses: Celgene; Roche; Shire. The authors declare no conflict of interest.
Figures



References
-
- World Health Organization-International Agency for Research on Cancer (IARC) GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. [(accessed on 10 October 2018)]; Available online: http://gco.iarc.fr/today/home.
-
- SEER Cancer Statistics Factsheets: Pancreatic Cancer National Cancer Institute. [(accessed on 12 October 2018)]; Available online: http://seer.cancer.gov/statfacts/html/pancreas.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

