Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium
- PMID: 31358049
- PMCID: PMC6664513
- DOI: 10.1186/s13287-019-1332-8
Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium
Abstract
Background: Intrauterine adhesion (IUA) is one of the most serious complications in patients with endometrial repair disorder after injury. Currently, there is no effective treatment for IUA. Stem cell is the main candidate of new therapy, which functions mainly through paracrine mechanism. Stem-derived exosomes (Exo) play an important role in tissue injury. Here, we mainly aim to study the effect of bone marrow mesenchymal stem cell (BMSC)-derived Exo on repairing endometrium of IUA animal models and its effect on TGF-β1 induced EMT in endometrial epithelial cells (EECs).
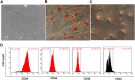
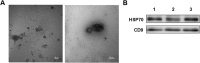
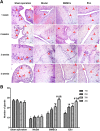
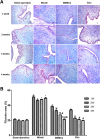
Methods: Totally, 64 female rabbits were randomly divided into Sham operation group, model group, BMSC treatment group, and Exo treatment group. EMT in EECs was induced by TGF-β1. Then, EECs were treated with Exo (25 μg/ml, 50 μg/ml, 100 μg/ml) for 24 h. HE staining and Masson staining were used to evaluate the changes in glandular number and fibrosis area. The expression levels of CK19 and VIM were detected by immunohistochemistry. Western blotting was used to detect the expression of CK19, VIM, FSP-1, E-cadherin, TGF-β1, TGF-β1R, Smad 2, and P-Smad 2. RT-PCR was used to detect mRNA expression levels of CK19, VIM, FSP-1, E-cadherin, TGF-β1, TGF-β1R, and Smad 2.
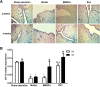
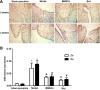
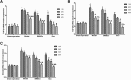
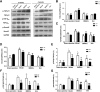
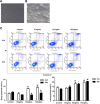
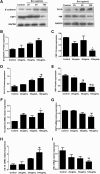
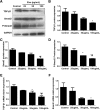
Results: Compared with the model group, the number of endometrial glands was significantly increased and endometrial fibrosis area was significantly decreased in BMSC and Exo groups (P < 0.05). CK19 level significantly increased whereas VIM level significantly decreased after treatment of BMSCs and Exo (P < 0.05). Additionally, the expressions of TGF-β1, TGF-β1R, and Smad2 mRNA were all significantly decreased after BMSC and Exo treatment (P < 0.05). Besides, phosphorylation levels of TGF-β1, TGF-β1R, and Smad2 were also significantly decreased in BMSC and Exo treatment groups (P < 0.05). Furthermore, there was no significant difference between BMSC and Exo treatment groups (P > 0.05). EMT was induced in EECs by 60 ng/ml TGF-β1 for 24 h. After Exo treatment for 24 h, mRNA expressions of CK-19 and E-cadherin increased, while those of VIM, FSP-1, TGF-β1, and Smad2 decreased. Additionally, protein expressions of CK-19 and E-cadherin increased, while those of VIM, FSP-1, TGF-β1, Smad2, and P-Smad2 decreased.
Conclusions: BMSC-derived Exo is involved in the repair of injured endometrium, with similar effect to that of BMSC, and can reverse EMT in rabbit EECs induced by TGF-β1. BMSC-derived Exo may promote endometrial repair by the TGF-β1/Smad signaling pathway.
Keywords: Eendometrial epithelial cell; Endometrium; Epithelial-mesenchymal transition; Exosomes; Intrauterine adhesion; Mesenchymal stem cell; TGF-β/Smad signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Metello JL, Jimenez JF. Intrauterine adhesions: etiopathogenesis. 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

