Receptor Heterodimerization Modulates Endocytosis through Collaborative and Competitive Mechanisms
- PMID: 31358286
- PMCID: PMC6712433
- DOI: 10.1016/j.bpj.2019.07.012
Receptor Heterodimerization Modulates Endocytosis through Collaborative and Competitive Mechanisms
Abstract
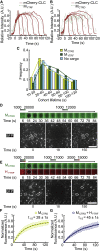
Recruitment of receptors into clathrin-coated structures is essential to signal transduction and nutrient uptake. Among the many receptors involved in these processes, a significant fraction forms dimers. Dimerization of identical partners has generally been thought to promote receptor recruitment for uptake because of increased affinity of the dimer for the endocytic machinery. But what happens when receptors with substantially different affinities for the endocytic machinery come together to form a heterodimer? Evidence from diverse receptor classes, including G-protein-coupled receptors and receptor tyrosine kinases, suggests that heterodimerization with a strongly recruited receptor can drive significant recruitment of a receptor that lacks direct interactions with the endocytic machinery. However, a systematic biophysical understanding of this effect has yet to be established. Motivated by the potential of such events to influence cell signaling, here, we investigate the impact of receptor heterodimerization on endocytic recruitment using a family of engineered model receptors. As expected, we find that dimerization of a weakly recruited receptor with a strongly recruited receptor promotes incorporation of the weakly recruited receptor to endocytic structures. However, the effectiveness of this collaborative mechanism depends heavily on the relative strengths of endocytic recruitment of the two receptors that make up the dimer. Specifically, as the strength of endocytic recruitment of the weakly recruited receptor approaches that of the strongly recruited receptor, monomers of each receptor compete with heterodimers for space within endocytic structures. In this regime, the presence of the strongly recruited receptor drives a reduction in incorporation of the weakly recruited receptor into clathrin-coated structures. Similarly, as the strength of the dimer bond between the two receptors is progressively weakened, competition begins to dominate over collaboration. Collectively, these results demonstrate that the impact of receptor heterodimerization on endocytic recruitment is controlled by a delicate balance between collaborative and competitive mechanisms.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Davis C.G., Lehrman M.A., Goldstein J.L. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986;45:15–24. - PubMed
-
- Vieira A.V., Lamaze C., Schmid S.L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. - PubMed
-
- Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

