Encoding of the Intent to Drink Alcohol by the Prefrontal Cortex Is Blunted in Rats with a Family History of Excessive Drinking
- PMID: 31358511
- PMCID: PMC6712204
- DOI: 10.1523/ENEURO.0489-18.2019
Encoding of the Intent to Drink Alcohol by the Prefrontal Cortex Is Blunted in Rats with a Family History of Excessive Drinking
Abstract
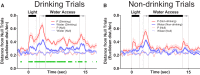
The prefrontal cortex (PFC) plays a central role in guiding decision making, and its function is altered by alcohol use and an individual's innate risk for excessive alcohol drinking. The primary goal of this work was to determine how neural activity in the PFC guides the decision to drink. Towards this goal, the within-session changes in neural activity were measured from medial PFC (mPFC) of rats performing a drinking procedure that allowed them to consume or abstain from alcohol in a self-paced manner. Recordings were obtained from rats that either lacked or expressed an innate risk for excessive alcohol intake, Wistar or alcohol-preferring (P) rats, respectively. Wistar rats exhibited patterns of neural activity consistent with the intention to drink or abstain from drinking, whereas these patterns were blunted or absent in P rats. Collectively, these data indicate that neural activity patterns in mPFC associated with the intention to drink alcohol are influenced by innate risk for excessive alcohol drinking. This observation may indicate a lack of control over the decision to drink by this otherwise well-validated supervisory brain region.
Keywords: alcohol-associated cues; alcohol-preferring rat; electrophysiology; information theory; neural encoding; prefrontal cortex.
Copyright © 2019 Linsenbardt et al.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
