A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus
- PMID: 31358625
- PMCID: PMC6697817
- DOI: 10.1073/pnas.1904700116
A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus
Abstract
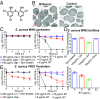
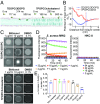
Treatment of Staphylococcus aureus infections is complicated by the development of antibiotic tolerance, a consequence of the ability of S. aureus to enter into a nongrowing, dormant state in which the organisms are referred to as persisters. We report that the clinically approved anthelmintic agent bithionol kills methicillin-resistant S. aureus (MRSA) persister cells, which correlates with its ability to disrupt the integrity of Gram-positive bacterial membranes. Critically, bithionol exhibits significant selectivity for bacterial compared with mammalian cell membranes. All-atom molecular dynamics (MD) simulations demonstrate that the selectivity of bithionol for bacterial membranes correlates with its ability to penetrate and embed in bacterial-mimic lipid bilayers, but not in cholesterol-rich mammalian-mimic lipid bilayers. In addition to causing rapid membrane permeabilization, the insertion of bithionol increases membrane fluidity. By using bithionol and nTZDpa (another membrane-active antimicrobial agent), as well as analogs of these compounds, we show that the activity of membrane-active compounds against MRSA persisters positively correlates with their ability to increase membrane fluidity, thereby establishing an accurate biophysical indicator for estimating antipersister potency. Finally, we demonstrate that, in combination with gentamicin, bithionol effectively reduces bacterial burdens in a mouse model of chronic deep-seated MRSA infection. This work highlights the potential repurposing of bithionol as an antipersister therapeutic agent.
Keywords: MRSA; bacterial persister; drug repurposing; membrane selectivity; membrane-active antimicrobials.
Conflict of interest statement
Conflict of interest statement: F.M.A. and E.M. have financial interests in Genma Biosciences and Octagon Therapeutics, companies that are engaged in developing antimicrobial compounds. E.M.’s and F.M.A.’s interests were reviewed and are managed by Rhode Island Hospital (E.M.) and Massachusetts General Hospital and Partners HealthCare (F.M.A.) in accordance with their conflict of interest policies. The remaining authors declare no competing financial interests.
Figures


References
-
- Lee A. S., et al. , Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 4, 18033 (2018). - PubMed
-
- Lewis K., Persister cells. Annu. Rev. Microbiol. 64, 357–372 (2010). - PubMed
-
- Conlon B. P., et al. , Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 1, 16051 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

