Energy conversion via metal nanolayers
- PMID: 31358629
- PMCID: PMC6697787
- DOI: 10.1073/pnas.1906601116
Energy conversion via metal nanolayers
Abstract
Current approaches for electric power generation from nanoscale conducting or semiconducting layers in contact with moving aqueous droplets are promising as they show efficiencies of around 30%, yet even the most successful ones pose challenges regarding fabrication and scaling. Here, we report stable, all-inorganic single-element structures synthesized in a single step that generate electrical current when alternating salinity gradients flow along its surface in a liquid flow cell. Nanolayers of iron, vanadium, or nickel, 10 to 30 nm thin, produce open-circuit potentials of several tens of millivolt and current densities of several microA cm-2 at aqueous flow velocities of just a few cm s-1 The principle of operation is strongly sensitive to charge-carrier motion in the thermal oxide nanooverlayer that forms spontaneously in air and then self-terminates. Indeed, experiments suggest a role for intraoxide electron transfer for Fe, V, and Ni nanolayers, as their thermal oxides contain several metal-oxidation states, whereas controls using Al or Cr nanolayers, which self-terminate with oxides that are redox inactive under the experimental conditions, exhibit dramatically diminished performance. The nanolayers are shown to generate electrical current in various modes of application with moving liquids, including sliding liquid droplets, salinity gradients in a flowing liquid, and in the oscillatory motion of a liquid without a salinity gradient.
Keywords: electron transfer; energy conversion; inorganic nanomaterials; solid–liquid interface; sustainability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

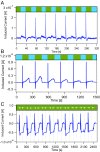
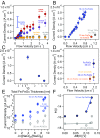


References
-
- Ghosh S., Sood A. K., Kumar N., Carbon nanotube flow sensors. Science 299, 1042–1044 (2003). - PubMed
-
- Zhang Z., et al. , Emerging hydrovoltaic technology. Nat. Nanotechnol. 13, 1109–1119 (2018). - PubMed
-
- Yin J., et al. , Generating electricity by moving a droplet of ionic liquid along graphene. Nat. Nanotechnol. 9, 378–383 (2014). - PubMed
-
- Yang S., et al. , Mechanism of electric power generation from ionic droplet motion on polymer supported graphene. J. Am. Chem. Soc. 140, 13746–13752 (2018). - PubMed
-
- Tang Q., Yang P., The era of water-enabled electricity generation from graphene. J. Mater. Chem. A Mater. Energy Sustain. 4, 9730–9738 (2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

