Development of bifunctional organocatalysts and application to asymmetric total synthesis of naucleofficine I and II
- PMID: 31358765
- PMCID: PMC6662887
- DOI: 10.1038/s41467-019-11382-8
Development of bifunctional organocatalysts and application to asymmetric total synthesis of naucleofficine I and II
Abstract
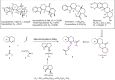
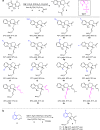
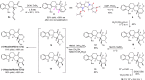
The proline-type organocatalysts has been efficiently employed to catalyze a wide range of asymmetric transformations; however, there are still many synthetically useful and challenging transformations that remain unachievable in an asymmetric fashion. Herein, a chiral bifunctional organocatalyst with a spirocyclic pyrrolidine backbone-derived containing fluoro-alkyl and aryl sulfonamide functionalities, are designed, prepared, and examined in the asymmetric Mannich/acylation/Wittig reaction sequence of 3,4-dihydro-β-carboline with acetaldehyde, acyl halides, and Wittig reagents. As a result, the spirocyclic pyrrolidine trifluoromethanesulfonamide catalyst can facilitate this versatile sequence as demonstrated by 18 examples displaying excellent enantioselectivity (up to 94% ee), as well as moderate to good yields (up to 54% over 3 steps). As a practical application, the asymmetric total synthesis of naucleofficine I (1a) and II (1b) in ten steps have been accomplished.
Conflict of interest statement
The authors declare no competing interests.
Figures
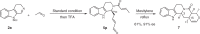
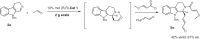
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

