Host circadian rhythms are disrupted during malaria infection in parasite genotype-specific manners
- PMID: 31358780
- PMCID: PMC6662749
- DOI: 10.1038/s41598-019-47191-8
Host circadian rhythms are disrupted during malaria infection in parasite genotype-specific manners
Abstract
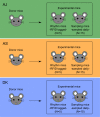
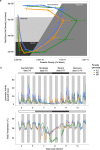
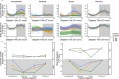
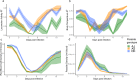
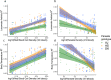
Infection can dramatically alter behavioural and physiological traits as hosts become sick and subsequently return to health. Such "sickness behaviours" include disrupted circadian rhythms in both locomotor activity and body temperature. Host sickness behaviours vary in pathogen species-specific manners but the influence of pathogen intraspecific variation is rarely studied. We examine how infection with the murine malaria parasite, Plasmodium chabaudi, shapes sickness in terms of parasite genotype-specific effects on host circadian rhythms. We reveal that circadian rhythms in host locomotor activity patterns and body temperature become differentially disrupted and in parasite genotype-specific manners. Locomotor activity and body temperature in combination provide more sensitive measures of health than commonly used virulence metrics for malaria (e.g. anaemia). Moreover, patterns of host disruption cannot be explained simply by variation in replication rate across parasite genotypes or the severity of anaemia each parasite genotype causes. It is well known that disruption to circadian rhythms is associated with non-infectious diseases, including cancer, type 2 diabetes, and obesity. Our results reveal that disruption of host circadian rhythms is a genetically variable virulence trait of pathogens with implications for host health and disease tolerance.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

