Distinct prognostic value of circulating anti-telomerase CD4+ Th1 immunity and exhausted PD-1+/TIM-3+ T cells in lung cancer
- PMID: 31358938
- PMCID: PMC6738094
- DOI: 10.1038/s41416-019-0531-5
Distinct prognostic value of circulating anti-telomerase CD4+ Th1 immunity and exhausted PD-1+/TIM-3+ T cells in lung cancer
Abstract
Background: Despite the critical roles of Th1-polarised CD4+ T cells in cancer immunosurveillance, the translation of their potential to clinical use remains challenging. Here, we investigate the clinical relevance of circulating antitumor Th1 immunity in non-small cell lung cancer (NSCLC).
Methods: The circulating antitumor Th1 response was assessed by the ELISpot assay in 170 NSCLC patients using a mixture of HLA class II-restricted peptides from telomerase (TERT). Phenotyping of blood immune cells was performed by flow cytometry.
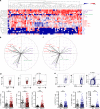
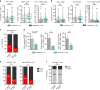
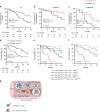
Results: TERT-reactive CD4 T-cell response was detected in 35% of NSCLC patients before any treatment. Functional analysis showed that these cells were effector memory and Th1 polarised capable to produce effector cytokines, such as IFN-γ, TNF-α and IL-2. The presence of anti-TERT Th1 response was inversely correlated with the level of exhausted PD-1+/TIM-3+CD4 T cells. The level of these two immune parameters differentially affected the survival, so that increased level of anti-TERT Th1 response and low rate of exhausted PD-1+TIM-3+CD4+ T cells were associated with a better prognosis.
Conclusions: Systemic anti-TERT Th1 response plays a strong antitumor protective role in NSCLC. This study underlines the potential interest of monitoring circulating antitumor Th1 response for patients' stratification and therapy decision.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. r. 2008;222:44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

