Tracing the cellular dynamics of sebaceous gland development in normal and perturbed states
- PMID: 31358966
- PMCID: PMC6978139
- DOI: 10.1038/s41556-019-0362-x
Tracing the cellular dynamics of sebaceous gland development in normal and perturbed states
Abstract
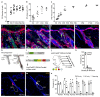
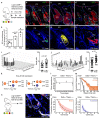
The sebaceous gland (SG) is an essential component of the skin, and SG dysfunction is debilitating1,2. Yet, the cellular bases for its origin, development and subsequent maintenance remain poorly understood. Here, we apply large-scale quantitative fate mapping to define the patterns of cell fate behaviour during SG development and maintenance. We show that the SG develops from a defined number of lineage-restricted progenitors that undergo a programme of independent and stochastic cell fate decisions. Following an expansion phase, equipotent progenitors transition into a phase of homeostatic turnover, which is correlated with changes in the mechanical properties of the stroma and spatial restrictions on gland size. Expression of the oncogene KrasG12D results in a release from these constraints and unbridled gland expansion. Quantitative clonal fate analysis reveals that, during this phase, the primary effect of the Kras oncogene is to drive a constant fate bias with little effect on cell division rates. These findings provide insight into the developmental programme of the SG, as well as the mechanisms that drive tumour progression and gland dysfunction.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Zouboulis CC, et al. Beyond acne: Current aspects of sebaceous gland biology and function. Rev Endocr Metab Disord. 2016;17:319–334. - PubMed
-
- Paus R, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

